Cat.NO.:A377823 Purity:95%
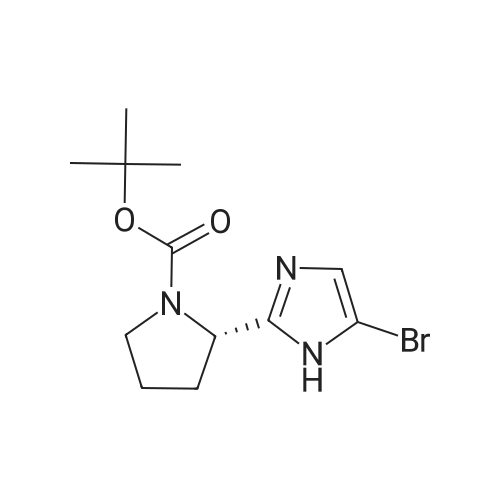
Product Details of [ 1007882-59-8 ]
| CAS No. : | 1007882-59-8 |
| Formula : |
C12H18BrN3O2 |
| M.W : |
316.19
|
| SMILES Code : | O=C(N1[C@H](C2=NC=C(Br)N2)CCC1)OC(C)(C)C |
| MDL No. : | MFCD16620748 |
| InChI Key : | GAZHEEFWONCMGH-QMMMGPOBSA-N |
| Pubchem ID : | 56605192 |
Safety of [ 1007882-59-8 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of [ 1007882-59-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1007882-59-8 ]
[ 1007882-59-8 ] Synthesis Path-Downstream 1~2
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| To a solution of compound Int-lla (0.5 g, 1.58 mmol, prepared as described in U.S. Patent Publication No. US20090068140) in DME (15 mL) at room temperature under N2 was added PdCl2(dppf)2 (258 mg, 0.30 mmol). The reaction mixture was allowed to stir at 100 0C for 5 minutes, then a solution of compound Int-llb (592 mg, 3.16 mmol) and K2CO3 (654 mg, 4.74 mmol) in 15 mL H2O was added to the reaction mixture in 3 portions over 10 minutes. The resulting reaction was allowed to stir for an additional 30 minutes, after which time thin-layer chromatography analysis indicated consumption of compound Int-7a. The reaction was allowed to stir for an additional 30 minutes, then was concentrated in vacuo, and the residue obtained was taken up in 150 mL ethyl acetate. The organic phase was separated, washed with water (50 mL), brine and dried over sodium sulfate. After filtration, the organic layer was concentrated in vacuo and the resulting residue was purified using flash liquic chromatography (0% to 100% EtOAc/Hexane) to provide 600 mg of compound Int-llc (>; 85% purity, theory 597 mg). HPLC (C18 column Gemini 5u HOA, 150X21.2 mm, 5 micron). FABMS: MH+ = 379 | ||
| To a solution of Compound Int-7d (0.5 g, 1.58 mmol) in DME (15 mL) at room temperature under N2 was added PdCl2(dppf)2 (258 mg, 0.30 mmol). The reaction mixture was allowed to stir at 100 0C for 5 minutes, then a solution of compound Int-19a (592 mg, 3.16 mmol) and K2CO3 (654 mg, 4.74 mmol) in 15 mL H2O was added to the reaction mixture in 3 portions over 10 minutes. The resulting reaction was allowed to stir for an additional 30 minutes, after which time thin-layer chromatography analysis indicated consumption of compound Int-7a. The reaction was allowed to stir for an additional 30 minutes, then was concentrated in vacuo, and the residue obtained was taken up in 150 niL ethyl acetate. The organic phase was separated, washed with water (50 rnL), brine and dried over sodium sulfate. After filtration, the organic layer was concentrated in vacuo and the resulting residue was purified using flash liquic chromatography (0% to 100% EtOAc/Hexane) to provide 600 mg of compound Int-19b (>; 85% purity, theory 597 mg). HPLC (C18 column Gemini 5u HOA, 150X21.2 mm, 5 micron). FABMS: MH+ = 379 |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| EXAMPLE 15 Preparation of Intermediate Compound Int-15c (0321) (0322) To a solution of compound Int-7d (0.5 g, 1.58 mmol) in DME (15 mL) at room temperature under N2 was added PdCl2(dppf)2 (258 mg, 0.30 mmol). The reaction mixture was allowed to stir at 100 C for 5 minutes, then a solution of compound Int-15b (592 mg, 3.16 mmol) and K2CO3 (654 mg, 4.74 mmol) in 15 mL H2O was added to the reaction mixture in 3 portions over 10 minutes. The resulting reaction was allowed to stir for an additional 30 minutes, after which time thin-layer chromatography analysis indicated consumption of compound Int-7a. The reaction was allowed to stir for an additional 30 minutes, then was concentrated in vacuo, and the resulting residue was taken up in 150 mL ethyl acetate. The organic phase was separated, washed with water (50 mL), brine and dried over sodium sulfate. After filtration, the organic layer was concentrated in vacuo and the resulting residue was purified using flash liquid chromatography (0% to 100% EtOAc/Hexane) to provide 600 mg of compound Int-15c (> 85% purity, theory 597 mg). HPLC (C18 column Gemini 5u 110A, 150X21.2 mm, 5 micron). FABMS: MH+ = 379 |







Reviews
There are no reviews yet.