Cat.NO.:A161565 Purity:99%
Product Details of [ 100306-33-0 ]
| CAS No. : | 100306-33-0 |
| Formula : |
C9H11ClO |
| M.W : |
170.64
|
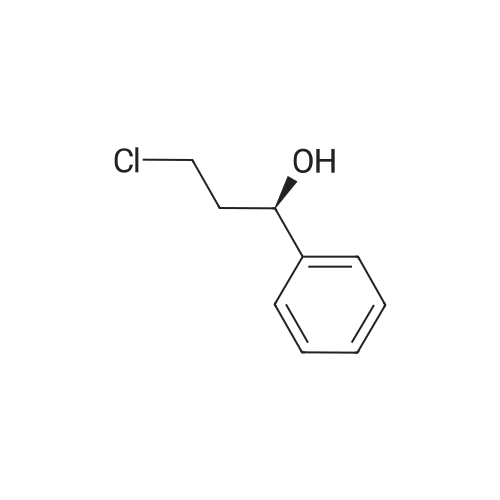
| SMILES Code : | O[C@@H](C1=CC=CC=C1)CCCl |
| MDL No. : | MFCD00075128 |
| InChI Key : | JZFUHAGLMZWKTF-SECBINFHSA-N |
| Pubchem ID : | 642409 |
Safety of [ 100306-33-0 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Computational Chemistry of [ 100306-33-0 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 11 |
| Num. arom. heavy atoms | 6 |
| Fraction Csp3 | 0.33 |
| Num. rotatable bonds | 3 |
| Num. H-bond acceptors | 1.0 |
| Num. H-bond donors | 1.0 |
| Molar Refractivity | 46.98 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
20.23 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
1.84 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
2.02 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
2.02 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
2.49 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
2.67 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
2.21 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-2.38 |
| Solubility | 0.718 mg/ml ; 0.00421 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-2.07 |
| Solubility | 1.45 mg/ml ; 0.00847 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-3.29 |
| Solubility | 0.0875 mg/ml ; 0.000513 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
Yes |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-5.91 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
2.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
1.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.59 |
Application In Synthesis of [ 100306-33-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100306-33-0 ]
[ 100306-33-0 ] Synthesis Path-Downstream 1~5
- 1
 [ 135748-35-5 ]
[ 135748-35-5 ]
 [ 100306-33-0 ]
[ 100306-33-0 ]
 [ 765-30-0 ]
[ 765-30-0 ]
- 4-Chloro-2-[(1R)-3-(cyclopropylamino)-1-phenylpropyl]oxy}-5-fluorobenzonitrile oxalate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With sodium iodide; oxalic acid; triethylamine; In tetrahydrofuran; N-methyl-acetamide; methanol; water; mineral oil; | EXAMPLE 25 4-Chloro-2-[(1R)-3-(cyclopropylamino)-1-phenylpropyl]oxy}-5-fluorobenzonitrile oxalate (R)-alpha-(2-Chloroethyl)benzenemethanol (341 mg, 2 mmol) was dissolved in tetrahydrofuran (10 ml) and treated with sodium hydride as a 60% suspension in mineral oil (480 mg, 3 mmol) followed after 10 minutes by <strong>[135748-35-5]4-chloro-2,5-difluorobenzonitrile</strong> (347 mg, 2 mmol). The mixture was stirred at room temperature for 18 h before being treated with methanol (1 ml) and then water (10 ml). The tetrahydrofuran was then removed via heating the vessel to 80 C. and applying a nitrogen stream. Once the tetrahydrofuran was evaporated the residue was extracted into dichloromethane, dried over magnesium sulphate and concentrated in vacuo. The resultant material was re-dissolved into dimethylformamide (8 ml) and treated with sodium iodide (305 mg, 2.03 mmol), triethylamine (565 mul, 4.06 mmol) and cyclopropylamine (114 mg, 2 mmol) before being heated to 60 C. for 5 days. The mixture was filtered and purified via RPHPLC on the crude reaction material. The purified compound was then treated with 50% saturated oxalic acid in ether to produce 74 mg of a white powder that was collected via filtration. MS APCI+ve m/z 345/347 [(M+H)+]. 1H NMR 400 MHz (d6-DMSO) 7.97-7.87 (m, 1H), 7.53-7.25 (m, 6H), 5.69 (m, 1H), 3.28-3.07 (m, 2H), 2.80-2.68 (m, 1H), 2.45-2.29 (m, 1H), 2.29-2.12 (m, 1H), 0.85-0.74 (m, 4H). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With yeast strain Aureobasidium pullulans CQA; at 28℃; for 48h;Microbiological reaction; Enzymatic reaction; | General procedure: Fresh plates of each yeast strain were streaked from the frozen stock in PDA. A single colony was used to inoculate 100mL of YM Broth. The culture was incubated at 28C and 150rpm for 48h and the cells were collected by centrifugation at 4000rpm and 4C for 15min. The pellet was washed three times with 50mL physiological serum. Afterward, 2g of yeast cells (wet weight) were suspended in 20mL of 10% dextrose solution and 30mg of the appropriate substrate were added. The culture was incubated at 28C and 150rpm in an orbital shaker ZHICHENG ZHWY-211B for 48h. | |
| 30%Chromat.; 23%Chromat. | With yeast culture of Candida viswanathii KCh 120; In acetone; at 25℃; for 6h;Microbiological reaction; | General procedure: Erlenmeyer flasks (300 ml), each containing 100 ml of the mediumconsisting of 3 g glucose and 1 g aminobac dissolved in water,were inoculated with a suspension of microorganisms and then incubated for 3-7 days at 25 C on a rotary shaker (190 rpm). After full growth of the culture 20 mg of a substrate dissolved in 1 ml of acetone was added. After 1, 3, 6, 9, 12 h and 1, 3, 6, 9 days of incubation under the above conditions, portions of 5 ml of the transformation mixture were taken out and extracted with CHCl3(3*10 ml). The extracts were dried over MgSO4, concentrated in vacuo, and analyzed by GC. All the experiments were repeatedthree times. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 21%Chromat. | With yeast culture of Aphanocladium album KCh 417; In acetone; at 25℃; for 144h;Microbiological reaction; | General procedure: Erlenmeyer flasks (300 ml), each containing 100 ml of the mediumconsisting of 3 g glucose and 1 g aminobac dissolved in water,were inoculated with a suspension of microorganisms and then incubated for 3-7 days at 25 C on a rotary shaker (190 rpm). After full growth of the culture 20 mg of a substrate dissolved in 1 ml of acetone was added. After 1, 3, 6, 9, 12 h and 1, 3, 6, 9 days of incubation under the above conditions, portions of 5 ml of the transformation mixture were taken out and extracted with CHCl3(3*10 ml). The extracts were dried over MgSO4, concentrated in vacuo, and analyzed by GC. All the experiments were repeatedthree times. |
| 33%Chromat. | With yeast culture of Saccharomyces cerevisiae KCh 464; In acetone; at 25℃; for 144h;Microbiological reaction; | General procedure: Erlenmeyer flasks (300 ml), each containing 100 ml of the mediumconsisting of 3 g glucose and 1 g aminobac dissolved in water,were inoculated with a suspension of microorganisms and then incubated for 3-7 days at 25 C on a rotary shaker (190 rpm). After full growth of the culture 20 mg of a substrate dissolved in 1 ml of acetone was added. After 1, 3, 6, 9, 12 h and 1, 3, 6, 9 days of incubation under the above conditions, portions of 5 ml of the transformation mixture were taken out and extracted with CHCl3(3*10 ml). The extracts were dried over MgSO4, concentrated in vacuo, and analyzed by GC. All the experiments were repeatedthree times. |
| 33%Chromat. | With yeast culture of Saccharomyces pastorianus KCh 906; In acetone; at 25℃; for 24h;Microbiological reaction; | General procedure: Erlenmeyer flasks (300 ml), each containing 100 ml of the mediumconsisting of 3 g glucose and 1 g aminobac dissolved in water,were inoculated with a suspension of microorganisms and then incubated for 3-7 days at 25 C on a rotary shaker (190 rpm). After full growth of the culture 20 mg of a substrate dissolved in 1 ml of acetone was added. After 1, 3, 6, 9, 12 h and 1, 3, 6, 9 days of incubation under the above conditions, portions of 5 ml of the transformation mixture were taken out and extracted with CHCl3(3*10 ml). The extracts were dried over MgSO4, concentrated in vacuo, and analyzed by GC. All the experiments were repeatedthree times. |









Reviews
There are no reviews yet.