Cat.NO.:A688787 Purity:97%
Product Details of [ 100929-33-7 ]
| CAS No. : | 100929-33-7 |
| Formula : |
C9H13NO2 |
| M.W : |
167.21
|
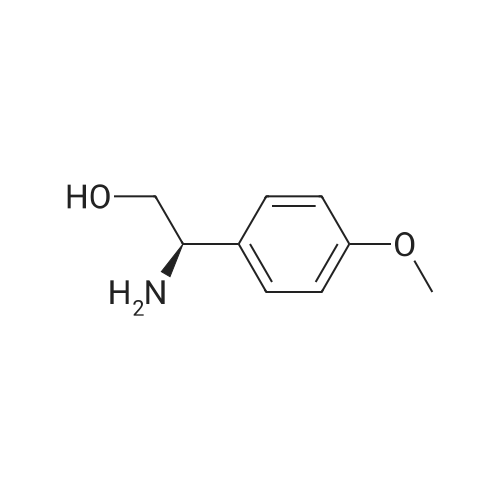
| SMILES Code : | COC1=CC=C([C@@H](N)CO)C=C1 |
| MDL No. : | MFCD09253729 |
| InChI Key : | OZNSMUSCZYUFHD-VIFPVBQESA-N |
| Pubchem ID : | 10866717 |
Safety of [ 100929-33-7 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H332-H335 |
| Precautionary Statements: | P280-P305+P351+P338-P310 |
Application In Synthesis of [ 100929-33-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100929-33-7 ]
[ 100929-33-7 ] Synthesis Path-Downstream 1~28
References: [1]Synthesis,1991,p. 996 – 998.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 52.71% | With lithium aluminium tetrahydride; In diethyl ether; for 3.0h;Reflux; | General procedure: Cryohydrate under bath, 2 – hydroxy -1 – phenyl ethyl oxime (4g, 26.5mmol) soluble in an amount of 40 ml anhydrous ethyl ether, is dropped into the containing lithium aluminum hydride (2.51g, 66 . 2mmol) ethyl ether suspension of 10 ml, then completing 30min, gradually heating to reflux, the reaction 3h. To be after the reaction is complete, under the ice salt bath, is dropped into the aqueous ether 2.5 ml, stirring 30min, then one by one 15% sodium hydroxide solution with 2.5 ml, continuing to stir 30min, add 7.5 ml water, stirring 1h, adding anhydrous magnesium sulfate, stirring 1h. Filtering, and for 20 ml of ethyl ether run filter washing cake. Drying of the organic layer with anhydrous magnesium sulfate, desolvation, yellow oily product obtained 0.58g, yield: 15.98%. |
References: [1]Synthesis,1991,p. 996 – 998.
References: [1]Synthesis,1991,p. 996 – 998.
References: [1]Synthesis,1991,p. 996 – 998.
References: [1]Synthesis,1991,p. 996 – 998.
References: [1]Synthesis,1991,p. 996 – 998.
References: [1]Synthesis,1991,p. 996 – 998.
- 10
 [ 590-42-1 ]
[ 590-42-1 ]
 [ 100929-33-7 ]
[ 100929-33-7 ]
- N-(tert-butyl)-N’-[1-(4-methoxyphenyl)-2-hydroxyethyl]thiourea [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| In diethyl ether; ethanol; water; | N-(tert-Butyl)-N’-[1-(4-methoxyphenyl)-2-hydroxyethyl]thiourea: The process is performed under the conditions of Example 1, starting with 1.3 g of 2-amino -2-(4-methoxyphenyl)-1-ethanol and 1.46 cm3 of tert-butyl isothiocyanate in 12 cm3 of absolute ethanol for 21 hours at a temperature in the region of 20 C. After concentration of the reaction mass under reduced pressure (5 kPa) at a temperature in the region of 40 C., the residue obtained is taken up in 10 cm3 of water. A crystalline precipitate forms, which is filtered off, washed with 3 times 5 cm3 of diethyl ether and dried under reduced pressure (10 Pa) at a temperature in the region of 40 C. 1.32 g of N-(tert-butyl) -N’-[1- (4-methoxyphenyl)-2-hydroxyethyl]thiourea are obtained in the form of a white solid melting at 127 C. 2-Amino-2-(4-methoxyphenyl)-1-ethanol: |
References: [1]Patent: US2002/19427,2002,A1 .
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With hydrogenchloride; In methanol; sodium hydroxide; sodium bicarbonate; | A mixture of 3.4 g of tert-butyl 1-(4-methoxyphenyl)-2-hydroxyethylcarbamate in 32 cm3 of methanol containing 10% by mass of anhydrous hydrogen chloride is stirred for 1 hour at a temperature in the region of 20 C. The reaction medium is concentrated under reduced pressure (5 kPa) at a temperature in the region of 40 C. The residue is taken up in 9 cm3 of aqueous 5% sodium hydrogen carbonate solution and the mixture is then extracted with 3 times 30 cm3 of dichloromethane. The aqueous phase is concentrated as above and the white solid obtained is then taken up in 17 cm3 of aqueous 1 N sodium hydroxide solution. The precipitate is extracted with 3 times 30 cm3 of dichloromethane. The combined organic extracts are evaporated under reduced pressure (5 kPa) at a temperature in the region of 40 C. The white solid obtained is dried under reduced pressure (10 Pa) at a temperature in the region of 40 C. 1.3 g of 2-amino-2-(4-methoxyphenyl)-1-ethanol are obtained in the form of a white solid melting at 96 C. tert-Butyl 1-(4-methoxyphenyl)-2-hydroxyethylcarbamate: |
References: [1]Patent: US2002/19427,2002,A1 .
References: [1]Patent: US2002/19427,2002,A1 .
References: [1]Synlett,2012,vol. 23,p. 1095 – 1098.
References: [1]Synlett,2012,vol. 23,p. 1095 – 1098.
References: [1]Synlett,2012,vol. 23,p. 1095 – 1098.
- 19
 [ 1265176-52-0 ]
[ 1265176-52-0 ]
 [ 100929-33-7 ]
[ 100929-33-7 ]
- 1-(2-hydroxy-1-(4-methoxyphenyl)ethyl)-3-(4-(1-(4-(trifluoromethoxy)phenyl)-1H-1,2,4-triazol-3-yl)phenyl)thiourea [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 81% | In tetrahydrofuran; at 20 – 24℃; | To 3-(4-isothiocyanato-phenyl)-l-(4-trifluoromethoxy-phenyl)-lH-l,2,4-triazole (1.09 g, 2.70 mmol) in tetrahydrofuran (7 mL) was added 2-amino-2-(4- methoxyphenyl)ethanol (0.506 g, 3.30 mmol) (Reggelin, M. et al., Synlett, 2012, 23, 1095-1098). The reaction was stirred overnight at room temperature. The reaction mixture was concentrated and the residue was recrystallized with petroleum ether providing the title compound as a white solid (1.42 g, 81%): *H NMR (300 MHz, CDCI3) delta 7.96 (s, 1H), 7.72 – 7.57 (m, 2H), 7.27 (s, 1H), 7.23 – 7.14 (m, 2H), 6.87 – 6.72 (m, 4H), 6.64 – 6.56 (m, 2H), 6.38 – 6.22 (m, 3H), 5.05 (s, 1H), 3.47 – 3.28 (m, 2H), 3.20 (s, 3H); ESIMS m/z 530 ([M + H]+). |
- 20
 [ 1265176-52-0 ]
[ 1265176-52-0 ]
 [ 100929-33-7 ]
[ 100929-33-7 ]
- 4-(4-methoxyphenyl)-N-(4-(1-(4-(trifluoromethoxy)phenyl)-1H-1,2,4-triazol-3-yl)phenyl)-4,5-dihydrothiazol-2-amine [ No CAS ]
References: [1]Patent: WO2015/200175,2015,A1 .
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 77%Spectr. | With 2,6-Di-tert-butyl-1,4-benzoquinone; oxygen; In ethanol; at 70℃; for 24.0h; | General procedure: To a solution of 2,6-di-tert-butyl-1,4-benzoquinone (2c, 0.20 mmol, 44 mg, 0.2 equiv) and p-anisidine (6a, 2.0 mmol, 246 mg, 2.0 equiv) in ethanol (3.2 mL) was added the 1,2-amino alcohol 1 (1.0 mmol). The flask was purged with O2 and then heated to 70 C with vigorous stirring for 24 h. After the reaction mixture was cooled to room temperature, benzyl ether (1.0 mmol, 190 muL, 1.0 equiv) was added as an internal standard and the reaction was concentrated under reduced pressure. 1H NMR was taken in CDCl3 and the spectrum obtained was used to assess the yield of imine 7. The desired imine product was subsequently purified by flash chromatography on triethylamine treated silica gel (eluent: 10% ethyl acetate in hexanes) to enable characterization of the imine product 7 through 1H NMR, 13C NMR, IR and MS. |








Reviews
There are no reviews yet.