Cat.NO.:A548447 Purity:98%
Product Details of [ 100-68-5 ]
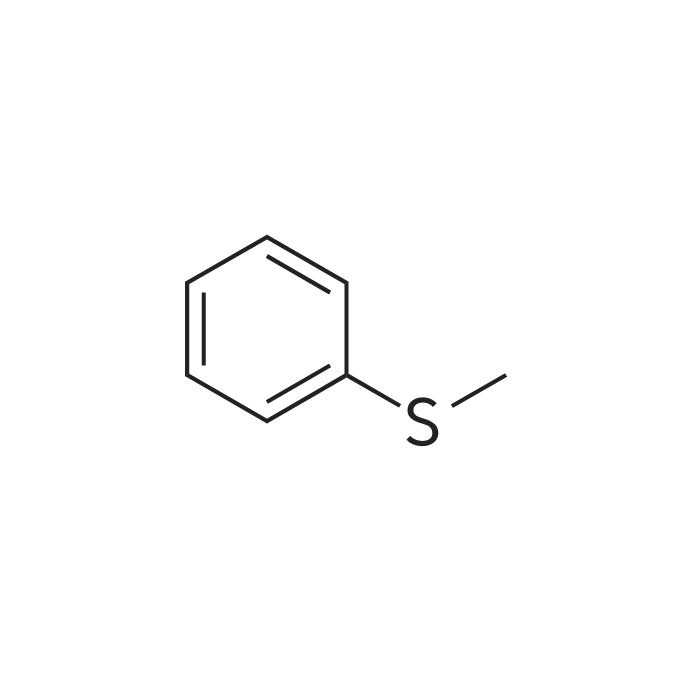
| CAS No. : | 100-68-5 |
| Formula : |
C7H8S |
| M.W : |
124.20
|
| SMILES Code : | CSC1=CC=CC=C1 |
| MDL No. : | MFCD00008559 |
| InChI Key : | HNKJADCVZUBCPG-UHFFFAOYSA-N |
| Pubchem ID : | 7520 |
Safety of [ 100-68-5 ]
| GHS Pictogram: |    |
| Signal Word: | Danger |
| Hazard Statements: | H227-H315-H318-H335-H410 |
| Precautionary Statements: | P210-P261-P264-P271-P273-P280-P302+P352-P304+P340-P305+P351+P338-P310-P321-P332+P313-P362-P370+P378-P391-P403+P233-P403+P235-P405-P501 |
| Class: | 9 |
| UN#: | 3334 |
| Packing Group: | Ⅲ |
Application In Synthesis of [ 100-68-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100-68-5 ]
[ 100-68-5 ] Synthesis Path-Downstream 1~3
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 48% | In carbon disulfide; | Step 1 Preparation of 1-(4-methylthiophenyl)-2-(4-fluorophenyl)ethanone; To a stirred solution of thioanisole (380 mL, 3.2 mol) and 4-fluorophenylacetyl chloride (300 g, 1.6 mol) in carbon disulfide (1.2 L), cooled to 5 C., was added anhydrous aluminum chloride portionwise at such a rate that the internal temperature did not rise above 15 C. The reaction was stirred at room temperature for 16 hours. The solution was cautiously poured into 2 L of ice and water. The aqueous solution was extracted with methylene chloride (6*150 mL), the combined extracts were dried over anhydrous MgSO4, filtered and concentrated in vacuo. The residue was dissolved in 800 mL of ether and cooled to 0 C. whereupon crystals of pure product formed which were isolated by filtration on a Buchner funnel and air dried to provide the ketone (199.6 g, 48%): mp 135-138 C. 1 H NMR (CDCl3 /TMS) 300 MHz 8.00 (d, J=8.7 Hz, 2H), 7.40-7.30 (m, 4H), 7.13-7.03 (m, 2H), 4.34 (s, 2H), 2.56 (s, 3H). Mass spectrum M+ =260. |
References: [1]Journal of Medicinal Chemistry,2004,vol. 47,p. 3874 – 3886.
[2]Phosphorus, Sulfur and Silicon and the Related Elements,2005,vol. 180,p. 1593 – 1600.
[3]Bioorganic and Medicinal Chemistry,2009,vol. 17,p. 558 – 568.
[4]Patent: US5668161,1997,A .
[5]Bioorganic and Medicinal Chemistry,2004,vol. 12,p. 1881 – 1893.
[2]Phosphorus, Sulfur and Silicon and the Related Elements,2005,vol. 180,p. 1593 – 1600.
[3]Bioorganic and Medicinal Chemistry,2009,vol. 17,p. 558 – 568.
[4]Patent: US5668161,1997,A .
[5]Bioorganic and Medicinal Chemistry,2004,vol. 12,p. 1881 – 1893.











Reviews
There are no reviews yet.