Cat.NO.:A115731 Purity:98%
Product Details of Ipatasertib
| CAS No. : | 1001264-89-6 |
| Formula : |
C24H32ClN5O2 |
| M.W : |
458.00
|
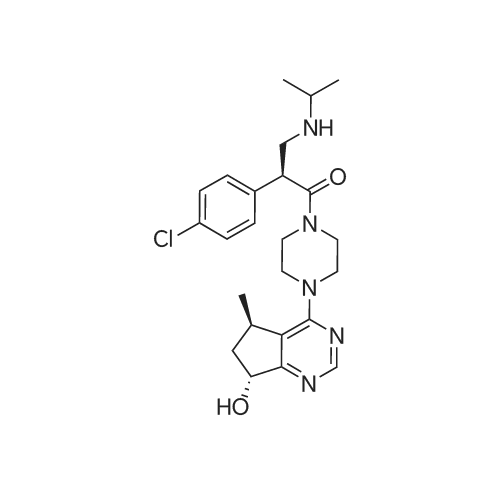
| SMILES Code : | O=C(N1CCN(C2=C([C@H](C)C[C@H]3O)C3=NC=N2)CC1)[C@@H](C4=CC=C(Cl)C=C4)CNC(C)C |
| Synonyms : |
GDC-0068; RG7440
|
| MDL No. : | MFCD22124514 |
| InChI Key : | GRZXWCHAXNAUHY-NSISKUIASA-N |
| Pubchem ID : | 24788740 |
Safety of Ipatasertib
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Related Pathways of Ipatasertib
- PI3K-AKT
Isoform Comparison
Biological Activity
| Target |
|
In Vitro:
| Concentration | Treated Time | Description | References |
| MOLM-13 | 75nM | 24 h | To validate selinexor’s ability to activate PI3K/AKT signaling, results showed increased AKT phosphorylation. | PMC9949365 |
| OCI-AML2 | 200nM | 24 h | To validate selinexor’s ability to activate PI3K/AKT signaling, results showed increased AKT phosphorylation. | PMC9949365 |
| PC3 cells | 500 nM | Inhibited translation of HGF, SPP1, and BGN | PMC11331482 | |
| Pten-sh TC1 cells | 500 nM | Inhibited translation of HGF, SPP1, and BGN | PMC11331482 | |
| BS-004 | 0.25–10 μM | 72 h | GDC-0068 showed only a minor reduction in cell viability in the PIK3CA-wildtype cell line BS-004. | PMC6827829 |
| MDA-MB-231 BrM2 | 0.25–10 μM | 72 h | GDC-0068 showed only a minor reduction in cell viability in the PIK3CA-wildtype cell line MDA-MB-231 BrM2. | PMC6827829 |
| JIMT-1 BR-3 | 0.25–10 μM | 72 h | GDC-0068 showed modest reduction in cell viability in the PIK3CA-mutant cell line JIMT-1 BR-3. | PMC6827829 |
| MDA-MB-453 | 0.25–10 μM | 72 h | GDC-0068 significantly decreased cell viability in the breast cancer cell line MDA-MB-453, with an IC50 of 0.322 μM. | PMC6827829 |
| MDA-MB-361 | 0.25–10 μM | 72 h | GDC-0068 significantly decreased cell viability in the PIK3CA-MT breast cancer brain metastatic cell line MDA-MB-361, with an IC50 of 2.83 μM. | PMC6827829 |
| LNCaP cells | 5 μM | To study the mechanism of ipatasertib resistance, it was found that resistant cells showed increased sensitivity to PIM inhibitors | PMC9019088 | |
| U87 cells | 1 mM | To evaluate the effect of Ipatasertib on the Nrf2 signaling pathway in IDH1-mutated and wild-type U87 cells, results showed that Ipatasertib suppressed the expression of Nrf2-related genes. | PMC10073324 | |
| NHA cells | 1 μM | To evaluate the effect of AKT inhibitor Ipatasertib on IDH1-mutated and wild-type NHA cells, results showed that IDH1-mutated cells were more sensitive to Ipatasertib. | PMC10073324 | |
| Hec-1B cells | 10 μM | 72 h | To investigate the inhibitory effect of Ipatasertib in combination with ascorbate on the proliferation of Hec-1B cells. Results showed that Ipatasertib alone significantly inhibited cell proliferation, and the combination with ascorbate produced a more potent inhibitory effect. | PMC11844297 |
| KLE cells | 10 μM | 72 h | To investigate the inhibitory effect of Ipatasertib in combination with ascorbate on the proliferation of KLE cells. Results showed that Ipatasertib alone significantly inhibited cell proliferation, and the combination with ascorbate produced a more potent inhibitory effect. | PMC11844297 |
| HCT116 | 10 μM | 0, 6, 12, and 24 h | To study the effect of Ipatasertib on the expression of Akt, FoxO3a, p65, and PUMA, the results showed that Ipatasertib significantly inhibited the phosphorylation of Akt, while activating FoxO3a and p65, and upregulating the expression of PUMA. | PMC6125489 |
| HCT116 | 10 μM | 12 and 24 h | To study the effect of Ipatasertib on colon cancer cell proliferation, the results showed that Ipatasertib significantly inhibited the proliferation of HCT116, p53−/−, and DLD1 cells, indicating that p53 is dispensable in this process. | PMC6125489 |
In Vivo:
| Administration | Dosage | Frequency | Description | References |
| Mice | MLL-AF9-driven AML model | Oral | 65mg/kg | Every other day, for five cycles | To evaluate the effect of ipatasertib combined with selinexor, results showed that the combination significantly prolonged the survival of mice. | PMC9949365 |
| Mice | Ptenpc−/−; Trp53pc−/− prostate cancer model | Oral | 100 mg/kg | Daily for six weeks | Inhibited tumor growth, reduced tumor-infiltrating PMN-MDSCs, and increased CD8+ T cells | PMC11331482 |
| Mice | PIK3CA-mutant breast cancer brain metastasis orthotopic xenograft model | Oral gavage | 100 mg/kg | Daily until the date of death of the last control mouse | In the PIK3CA-mutant MDA-MB-361 breast cancer brain metastasis model, GDC-0068 significantly inhibited tumor growth and significantly extended the survival of mice, with a median survival of 109 days compared to 82.5 days in the control group. | PMC6827829 |
| Mice | LNCaP prostate cancer xenograft model | Oral | 25 mg/kg | Once daily for 21 days | To evaluate the effect of combined treatment with ipatasertib and PIM inhibitors on resistant tumors, it was found that the combination significantly inhibited tumor growth | PMC9019088 |
| CB-17 Scid mice | TS603 xenograft model | Gavage | 40 mg/kg | Once on day 15 and day 30 | To evaluate the effect of Ipatasertib combined with TMZ on IDH-mutated TS603 xenograft model, results showed that the combination treatment significantly prolonged the survival of the mice. | PMC10073324 |
| Nude mice | Xenograft model | Oral | 30 mg/kg | Once daily for 15 consecutive days | To study the antitumor activity of Ipatasertib in vivo, the results showed that Ipatasertib significantly inhibited the growth of WT tumors, but had less effect on PUMA?/? tumors, indicating that PUMA is indispensable for the antitumor effect of Ipatasertib. | PMC6125489 |
Clinical Trial:
| NCT Number | Conditions | Phases | Recruitment | Completion Date | Locations |
| NCT03341884 | Hepatic Insufficiency | PHASE1 | COMPLETED | 2018-06-26 | Clinical Pharmacology of Miami… More >>, Inc., Miami, Florida, 33014, United States|New Orleans Center for Clinical Research, Knoxville, Tennessee, 37920, United States|American Research Corporation Inc., San Antonio, Texas, 78215, United States Less << |
| NCT04467801 | NSCLC Stage IV|NSCLC Stage III… More >>B Less << | PHASE2 | RECRUITING | 2025-08-25 | The University of Kansas Cance… More >>r Center (KUCC), Fairway, Kansas, 66205, United States|The University of Kansas Cancer Center, Westwood Campus, Kansas City, Kansas, 66205, United States|The University of Kansas Cancer Center, Overland Park Clinic, Overland Park, Kansas, 66210, United States|The University of Kansas Cancer Center, North Clinic, Kansas City, Missouri, 64154, United States|The University of Kansas Cancer Center, Lee’s Summit Clinic, Lee’s Summit, Missouri, 64064, United States Less << |
| NCT04341259 | Solid Tumors | PHASE1 | COMPLETED | 2023-04-06 | Fudan University Shanghai Canc… More >>er Center, Shanghai City, 200120, China Less << |
| NCT01090960 | Solid Cancers | PHASE1 | COMPLETED | 2025-02-15 | Barcelona, 08035, Spain|Valenc… More >>ia, 46010, Spain Less << |
Protocol
| Bio Calculators | ||||
| Preparing Stock Solutions |  |
1mg | 5mg | 10mg |
|
1 mM 5 mM 10 mM |
2.18mL 0.44mL 0.22mL |
10.92mL 2.18mL 1.09mL |
21.83mL 4.37mL 2.18mL |
|


Reviews
There are no reviews yet.