Cat.NO.:A690887 Purity:98%
Product Details of Fasiglifam
| CAS No. : | 1000413-72-8 |
| Formula : |
C29H32O7S |
| M.W : |
524.63
|
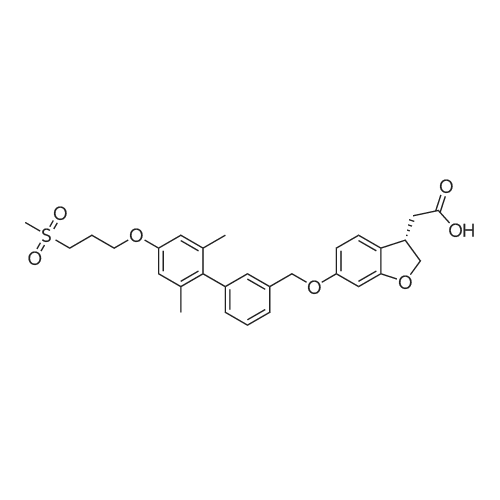
| SMILES Code : | O=C(O)C[C@@H]1COC2=CC(OCC3=CC(C4=C(C)C=C(OCCCS(=O)(C)=O)C=C4C)=CC=C3)=CC=C12 |
| Synonyms : |
Tak-875
|
| MDL No. : | MFCD18251445 |
| InChI Key : | BZCALJIHZVNMGJ-HSZRJFAPSA-N |
| Pubchem ID : | 24857286 |
Safety of Fasiglifam
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Related Pathways of Fasiglifam
- GPCR
Isoform Comparison
Biological Activity
In Vitro:
| Concentration | Treated Time | Description | References |
| HEK293 cells | 10 µM | 2 hours | Evaluate the binding ability of Fasiglifam to GPR40 receptor | PMC5917010 |
| CHO-GPR40 cells | 5 μM | 1 hour | To determine agonistic activity of compounds against FFA1 receptor, results showed compounds 4b and 4c activated 80% and 61% receptor activity at 5 μM concentration, respectively | PMC6021034 |
| Mouse colonic mucosal cells | 300 nM | 10–15 minutes | To compare the pharmacology of FFA1 and FFA4 agonists, finding selective agonists more potent than GW9508 | PMC5715575 |
| Human airway smooth muscle (HASM) cells | 10 µM | 30 min | TAK875 significantly attenuated histamine-induced MLC phosphorylation and cortical tension development, while GW9508 showed little effect. | PMC7708129 |
| RIN-m5f cells | 30 or 150 mg/mL | 48 hours | To evaluate the effect of YD on insulin secretion in high-glucose and high-fat injured RIN-m5f cells. Results showed that high concentration YD (150 mg/mL) significantly increased glucose-stimulated insulin secretion (GSIS) levels, similar to the TAK-875 group. | PMC10043368 |
| Human liver organoids (HLOs) | 3, 10, 30 μM | 7 days | To evaluate oxidative stress and immune responses induced by TAK-875. Results showed that TAK-875 treatment led to increased ROS formation and significant release of inflammatory cytokines MCP-1 and IL-6. | PMC9981852 |
In Vivo:
| Administration | Dosage | Frequency | Description | References |
| Mice | GPR40 knockout mice | Oral | 10 mg/kg | Single dose | Evaluate the effect of Fasiglifam on blood glucose and insulin levels | PMC5917010 |
| SD rats | Oral glucose tolerance test | Oral | 10 mg/kg | Single dose | Evaluate the effect of HWL-088 on glucose tolerance in rats | PMC7174891 |
| ZDF (fa/fa) rats | Type 2 diabetic rat model | Gavage | 10 mg/kg/day | Once daily for 10 weeks | To evaluate the effect of TAK-875 on blood glucose and insulin secretion in type 2 diabetic rats. Results showed that TAK-875 significantly reduced fasting blood glucose (FBG) and the area under the curve (AUC) of OGTT, and increased the AUC of insulin release test (IRT) and glucose-stimulated insulin secretion (GSIS). | PMC10043368 |
| Mice | Diabetes model | Intraperitoneal injection | 27 mg/kg | Single dose | To evaluate the effect of FFA1 agonist on blood glucose control, finding it improved glucose tolerance | PMC5715575 |
| Mice | Gpr40 knockout and wild-type mice | Oral | 30 mg/kg | Single dose, evaluated after 30 minutes | To assess the effect of GPR40 agonists on plasma GLP-1 and GIP levels. Results showed that Gq+Gs agonists like AM-1638 and AM-5262 significantly increased plasma GLP-1 and GIP levels. | PMC4314522 |
| Rats | Type 1 diabetes model rats | Oral | 5 and 10 mg/kg | Immediately before glucose load | TAK-875 (a GPR40 agonist) did not decrease postprandial blood glucose levels up to 30 min after glucose load. | PMC10424117 |
| Collaborative Cross mice | Collaborative Cross mice | Oral gavage | 600 mg/kg | Single dose | To evaluate the mechanisms of TAK-875-induced liver injury and genetic risk factors in Collaborative Cross mice. Results showed that a single high dose of TAK-875 did not cause overt liver injury, but gene expression profiling revealed transcriptional changes associated with immune response, bile acid homeostasis, oxidative stress, and mitochondrial dysfunction. | PMC8936092 |
Clinical Trial:
| NCT Number | Conditions | Phases | Recruitment | Completion Date | Locations |
| NCT01433393 | Diabetes Mellitus | Phase 3 | Completed | – | Japan
… More >> Kisarazu-shi, Chiba, Japan Matsuyama-shi, Ehime, Japan Fukuoka-shi Nishi-ku, Fukuoka, Japan Kasuga-shi, Fukuoka, Japan Naka-shi, Ibaragi, Japan Tsuchiura-shi, Ibaragi, Japan Takamatsu-shi, Kagawa, Japan Kyoto-shi Fushimi-ku, Kyoto, Japan Nagasaki-shi, Nagasaki, Japan Kashihara-shi, Nara, Japan Kashiwara-shi, Osaka, Japan Osaka-shi Tsurumi-ku, Osaka, Japan Sakai-shi Nishi-ku, Osaka, Japan Shimotsuke-shi, Tochigi, Japan Chiyoda-ku, Tokyo, Japan Itabashi-ku, Tokyo, Japan Ota-ku, Tokyo, Japan Shinjuku-ku, Tokyo, Japan Toshima-ku, Tokyo, Japan Less << |
| NCT01585792 | Diabetic Patients | Phase 3 | Completed | – | Japan
… More >> Fukuoka-shi, Fukuoka, Japan Sumida-ku, Tokyo, Japan Less << |
| NCT01007097 | Diabetes Mellitus, Type 2 | Phase 2 | Completed | – | – |
| NCT01433406 | Diabetes Mellitus | Phase 3 | Completed | – | – |
| NCT01414920 | Diabetes Mellitus, Type 2 | Phase 2 | Completed | – | – |
| NCT01433419 | Diabetes Mellitus | Phase 3 | Completed | – | Japan
… More >> Katori-shi, Chiba, Japan Niihama-shi, Ehime, Japan Chikushino-shi, Fukuoka, Japan Fukuoka-shi, Fukuoka, Japan Onga-gun, Fukuoka, Japan Yukuhashi-shi, Fukuoka, Japan Hiroshima-shi, Hiroshima, Japan Chitose-shi, Hokkaido, Japan Sapporo-shi, Hokkaido, Japan Koga-shi, Ibaragi, Japan Ushiku-shi, Ibaragi, Japan Chigasaki-shi, Kanagawa, Japan Kamakura-shi, Kanagawa, Japan Minamata-shi, Kumamoto, Japan Yatsushiro-shi, Kumamoto, Japan Sasebo-shi, Nagasaki, Japan Kashihara-shi, Nara, Japan Okinawa-shi, Okinawa, Japan Izumi-shi, Osaka, Japan Suita-shi, Osaka, Japan Fujimi-shi, Saitama, Japan Shimotsuga-gun, Tochigi, Japan Komatsushima-shi, Tokushima, Japan Bunkyo-ku, Tokyo, Japan Chiyoda-ku, Tokyo, Japan Mitaka-shi, Tokyo, Japan Shibuya-ku, Tokyo, Japan Shinjuku-ku, Tokyo, Japan Toshima-ku, Tokyo, Japan Shunan-shi, Yamaguchi, Japan Ube-shi, Yamaguchi, Japan Less << |
| NCT01456195 | Diabetes Mellitus, Type 2 | Phase 3 | Completed | – | – |
| NCT01549964 | Glycemic Control | Phase 3 | Terminated(Due to concerns abo… More >>ut potential liver safety (See Detailed Description)) Less << | – | – |
| NCT01834274 | Diabetes Mellitus, Type 2 | Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT01834274 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01982253 | Type 2 Diabetes Mellitus | Phase 2 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT01982253 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01481116 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01549964 | – | Terminated(Due to concerns abo… More >>ut potential liver safety (See Detailed Description)) Less << | – | – | |
| NCT02015780 | Type 2 Diabetes Mellitus … More >> Chronic Kidney Disease Less << |
Phase 3 | Withdrawn(Due to potential con… More >>cerns about liver safety (See Detailed Description)) Less << | January 2016 | – |
| NCT01481116 | Diabetes Mellitus, Type 2 | Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT01456195 | – | Completed | – | – | |
| NCT01829464 | Diabetes | Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT01829477 | Diabetes Mellitus, Type 2 | Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT00949091 | Diabetes Mellitus, Type 2 | Phase 1 | Completed | – | – |
| NCT01496443 | Pharmacokinetics | Phase 1 | Completed | – | United States, Texas … More >> San Antonio, Texas, United States Less << |
| NCT01829477 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01647542 | Type 2 Diabetes Mellitus | Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
| NCT01647542 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01609582 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01829464 | – | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – | |
| NCT01609582 | Type 2 Diabetes … More >> Cardiovascular Disease Less << |
Phase 3 | Terminated(Due to potential co… More >>ncerns about liver safety (See Detailed Description)) Less << | – | – |
Protocol
| Bio Calculators | ||||


Reviews
There are no reviews yet.