Cat.NO.:A358801 Purity:98%
Product Details of Daclatasvir
| CAS No. : | 1009119-64-5 |
| Formula : |
C40H50N8O6 |
| M.W : |
738.88
|
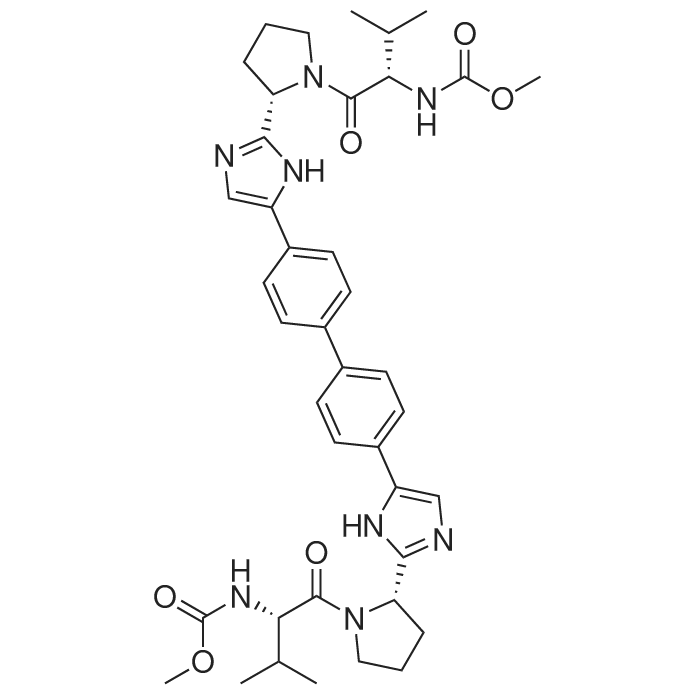
| SMILES Code : | O=C([C@@H](NC(OC)=O)C(C)C)N1CCC[C@H]1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C5=CN=C([C@@H]6CCCN6C([C@@H](NC(OC)=O)C(C)C)=O)N5)C=C4 |
| Synonyms : |
BMS-790052; EBP 883
|
| MDL No. : | MFCD17129086 |
| InChI Key : | OVPGYLMBPQNZNE-GJVQWJCYSA-N |
| Pubchem ID : | 66575053 |
Safety of Daclatasvir
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Isoform Comparison
Biological Activity
| Target |
|
In Vitro:
| Concentration | Treated Time | Description | References |
| Huh7 cells | 1 nM | 10 days | To evaluate the effect of Daclatasvir on HCV replication and assembly/secretion. Results showed that Daclatasvir treatment led to a decline in intracellular HCV RNA, similar to the polymerase inhibitor NM107, but extracellular HCV titers declined more rapidly, indicating its inhibitory effect on viral assembly/secretion. | PMC3593898 |
| primary RDEB dermal fibroblasts | 1 μM | 48 h | To evaluate the effect of Daclatasvir on TGF-β signaling pathway, results showed that Daclatasvir inhibited the expression of TGF-β pathway targets collagen I, phosphorylated AKT, and phosphorylated SMAD3. | PMC11018630 |
In Vivo:
| Administration | Dosage | Frequency | Description | References |
| Humanized-liver mice | HBV/HCV co-infection model | Oral | 30 mg/kg | Once a day for 4 weeks | To evaluate the effect of DAA treatment on HBV replication in HBV/HCV-co-infected mice. Results showed that HCV was eliminated after DAA treatment, and HBV replication was upregulated. | PMC6976581 |
| Alb-uPA/Scid mice | HCV genotype 1b or 3 infection model | Oral | 10 mg/kg | Once daily for 4 weeks | Evaluate the inhibitory effect and drug resistance of Daclatasvir monotherapy on HCV | PMC7677215 |
| Human hepatocyte chimeric TK-NOG mice | HCV infection model | Oral | 30 mg/kg | Once daily for 4 weeks | To assess the origin of HCV NS5A L31V-Y93H double substitutions after treatment failure with daclatasvir and asunaprevir combination therapy | PMC5278351 |
| Mice | RDEB mouse model | Oral (via drinking water) | 30 mg/kg | From conception to weaning (28 days) | To evaluate the effect of Daclatasvir on fibrosis and quality of life in RDEB mice, results showed that Daclatasvir significantly improved survival, increased weight and activity, and reduced pruritus-induced hair loss. Additionally, the expression of fibrosis markers phospho-SMAD3 and collagen I was reduced in the skin. | PMC11018630 |
Clinical Trial:
| NCT Number | Conditions | Phases | Recruitment | Completion Date | Locations |
| NCT02095860 | Hepatitis C | Phase 1 | Completed | – | United States, Texas … More >> Healthcare Discoveries, Llc D/B/A Icon Development Solutions San Antonio, Texas, United States, 78209 Less << |
| NCT02531269 | – | Completed | – | Switzerland
… More >> Local Institution Basel, Switzerland Less << |
|
| NCT03186313 | Hepatitis C | Phase 3 | Completed | – | Egypt
… More >> Egyptian Liver Hospital Shirbīn, Dakahlia, Egypt, 35681 Less << |
| NCT02170727 | Hepatitis C Virus | Phase 3 | Completed | – | Korea, Republic of … More >> Local Institution Busan, Korea, Republic of, 602-739 Local Institution Busan, Korea, Republic of, 614-735 Local Institution Gyeonggi-do, Korea, Republic of, 463-707 Local Institution Gyeonggi-Do, Korea, Republic of, 480-717 Local Institution Gyeongsangnam-do, Korea, Republic of, 626-770 Local Institution Inchoen, Korea, Republic of, 405-760 Local Institution Seoul, Korea, Republic of, 120-752 Local Institution Seoul, Korea, Republic of, 135-710 Local Institution Seoul, Korea, Republic of, 138-736 Local Institution Seoul, Korea, Republic of, 156-755 Local Institution Kazan, Russian Federation, 420140 Local Institution Moscow, Russian Federation, 109240 Local Institution Kaohsiung, Taiwan, 807 Local Institution Kaohsiung, Taiwan, 833 Local Institution Taichung, Taiwan, 40447 Local Institution Taichung, Taiwan, 40705 Local Institution Tainan, Taiwan, 704 Local Institution Taipei, Taiwan, 100 Local Institution Taipei, Taiwan, 112 Local Institution Taoyuan, Taiwan, 333 Less << |
| NCT02045966 | Hepatitis C | Phase 1 | Completed | – | – |
| NCT01016912 | Hepatitis C Infection | Phase 2 | Completed | – | Japan
… More >> Local Institution Hiroshima City, Hiroshima, Japan, 734-0037 Local Institution Sapporo-Shi, Hokkaido, Japan, 060-0033 Local Institution Kawasaki-Shi, Kanagawa, Japan, 2138587 Local Institution Suita-Shi, Osaka, Japan, 5650871 Local Institution Iruma-Gun, Saitama, Japan, 3500495 Local Institution Minato-Ku, Tokyo, Japan, 105-0001 Less << |
| NCT01573351 | Hepatitis C Virus | Phase 3 | Completed | – | – |
| NCT02727933 | – | Recruiting | September 30, 2020 | Korea, Republic of … More >> Local Institution Recruiting Seoul, Korea, Republic of |
|
| NCT02250001 | – | Completed | – | Japan
… More >> Local Institution Chuo-ku, Tokyo, Japan, 104-0033 Less << |
|
| NCT01016912 | – | Completed | – | – | |
| NCT02292966 | Hepatitis C, Chronic | Phase 4 | Withdrawn | June 2016 | Australia, New South Wales … More >> St Vincent’s Hospital Sydney, New South Wales, Australia, 2010 Westmead Hospital Westmead, New South Wales, Australia, 2145 Less << |
| NCT03500562 | – | Recruiting | December 31, 2021 | China, Beijing
… More >> Local Institution Beijing, Beijing, China, 100054 |
|
| NCT01741545 | Hepatitis C Virus | Phase 3 | Completed | – | – |
| NCT00983957 | – | Completed | – | – | |
| NCT02161939 | – | – | – | – | |
| NCT00983957 | Chronic Hepatitis C | Phase 1 | Completed | – | United States, Arizona … More >> MDS Pharma Services (US), Inc Tempe, Arizona, United States, 85283 Covance Clinical Research Unit, Inc. Austin, Texas, United States, 78752 Local Institution St. Laurent, Quebec, Canada, H4R2N6 Less << |
| NCT02175966 | Hepatitis C | Phase 2 | Completed | – | United States, California … More >> Inland Empire Liver Foundation Rialto, California, United States, 92377 Northwestern University Feinberg School Of Medicine Chicago, Illinois, United States, 60611 Indiana University Health – University Hospital Indianapolis, Indiana, United States, 46202 Johns Hopkins University Lutherville, Maryland, United States, 21093 Texas Liver Institute San Antonio, Texas, United States, 78215 Less << |
| NCT00874770 | Hepatitis C Infection | Phase 2 | Completed | – | United States, Alabama … More >> Alabama Liver & Digestive Specialists (Alds) Montgomery, Alabama, United States, 36116 University Of Colorado Denver & Hospital Aurora, Colorado, United States, 80045 Yale University School Of Medicine New Haven, Connecticut, United States, 06520 Mercy Medical Center Baltimore, Maryland, United States, 21202 Llc Dba The Research Institute Springfield, Massachusetts, United States, 01107 Veterans Affairs Medical Center Bronx, New York, United States, 10468 Carolinas Center For Liver Disease Statesville, North Carolina, United States, 28677 Options Health Research, Llc Tulsa, Oklahoma, United States, 74104 Healthcare Research Consultants Tulsa, Oklahoma, United States, 74135 North Texas Research Institute Arlington, Texas, United States, 76012 Metropolitan Research Fairfax, Virginia, United States, 22031 Local Institution Creteil, France, 94010 Local Institution Paris Cedex 14, France, 75679 Local Institution Vandoeuvre Les Nancy, France, 54511 Less << |
| NCT00874770 | – | Completed | – | – | |
| NCT01359644 | Chronic Hepatitis C | Phase 2 | Completed | – | United States, California … More >> Southern California Liver Centers Coronado, California, United States, 92118 Research And Education, Inc. San Diego, California, United States, 92105 University Of Colorado Denver & Hospital Aurora, Colorado, United States, 80045 University Of Florida Hepatology Gainesville, Florida, United States, 32610 Orlando Immunology Center Orlando, Florida, United States, 32803 Miami Research Associates South Miami, Florida, United States, 33143 Mercy Medical Center Baltimore, Maryland, United States, 21202 Johns Hopkins University Lutherville, Maryland, United States, 21093 University Of Michigan Health System Ann Arbor, Michigan, United States, 48109 Bronx Va Medical Center 3c Sub-J Bronx, New York, United States, 10468 Weill Cornell Medical College New York, New York, United States, 10021 Options Health Research, Llc Tulsa, Oklahoma, United States, 74104 Healthcare Research Consultants Tulsa, Oklahoma, United States, 74135 University Of Pennsylvania Philadelphia, Pennsylvania, United States, 19104 Alamo Medical Research San Antonio, Texas, United States, 78215 Metropolitan Research Annandale, Virginia, United States, 22003 Dean Clinic Madison, Wisconsin, United States, 53715 Local Institution San Juan, Puerto Rico, 00927 Less << |
| NCT00859053 | Hepatic Insufficiency | PHASE1 | COMPLETED | 2025-09-09 | Advanced Clinical Research Ins… More >>titute, Anaheim, California, 92801, United States|Orlando Clinical Research Center, Orlando, Florida, 32809, United States Less << |
| NCT01448044 | Hepatitis C | Phase 3 | Completed | – | United States, California … More >> Scti Research Foundation San Clemente, California, United States, 92673 Umass Memorial Medical Center Worcester, Massachusetts, United States, 01655 University Gastroenterology Providence, Rhode Island, United States, 02905 Metropolitan Research Annandale, Virginia, United States, 22003 Local Institution Bondy Cedex, France, 93143 Local Institution Creteil, France, 94000 Local Institution La Roche-Sur-Yon Cedex 9, France, 85925 Local Institution Marseille Cedex 08, France, 13285 Local Institution Nice Cedex 03, France, 06202 Local Institution Orleans Cedex 2, France, 45067 Local Institution Paris, France, 75013 Local Institution Paris, France, 75475 Local Institution Strasbourg Cedex, France, 67091 Local Institution Toulouse Cedex 09, France, 31059 Local Institution Villejuif, France, 94804 Local Institution Thesaloniki, Greece, 54639 Local Institution Roma, Italy, 00149 Local Institution Torino, Italy, 10126 Local Institution San Juan, Puerto Rico, 00927 Local Institution A Coruna, Spain, 15706 Local Institution Barcelona, Spain, 08003 Local Institution Barcelona, Spain, 08035 Local Institution Madrid, Spain, 28046 Local Institution London, Greater London, United Kingdom, SE5 9RS Local Institution Details |


Reviews
There are no reviews yet.