Cat.NO.:A377289 Purity:95%
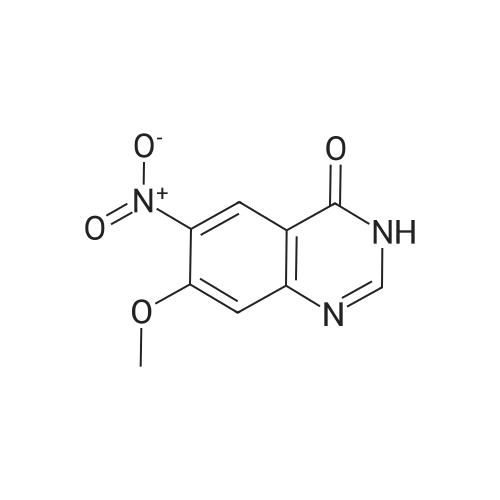
Product Details of [ 1012057-47-4 ]
| CAS No. : | 1012057-47-4 |
| Formula : |
C9H7N3O4 |
| M.W : |
221.17
|
| SMILES Code : | O=C1NC=NC2=C1C=C([N+]([O-])=O)C(OC)=C2 |
| MDL No. : | MFCD11869863 |
| InChI Key : | AJRGEVFKZSWGFX-UHFFFAOYSA-N |
| Pubchem ID : | 135547025 |
Safety of [ 1012057-47-4 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Application In Synthesis of [ 1012057-47-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1012057-47-4 ]
[ 1012057-47-4 ] Synthesis Path-Downstream 1~5
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 83% | With trichlorophosphate; for 4h;Heating / reflux; | Compound 0304 (3.8 g, 17.2 mmol) was suspended in POCI3 (75 mL), the mixture was heated to reflux for 4 hours. The additional POCI3 was removed in a vacuum. The residue was dissolved in a mixture of dichloromethane (50 mL) and aqueous NaHCCh (50 mL) .The organic layer was dried and the solvent was removed to give the title compound 0305 (3.4 g, 83%). 1H NMR (DMSO-J6): delta 4.05 (s, 3H), 7.44 (s, IH), 8.27 (s, IH), 8.53 (s, IH). |
| 83% | With trichlorophosphate; for 4h;Heating / reflux; | Step 22d. 4-Chloro-7-methoxy-6-nitroquinazoline (compound 0305) Compound 0304 (3.8 g, 17.2 mmol) was suspended in POCl3 (75 mL), the mixture was heated to reflux for 4 hours. The additional POCl3 was removed in a vacuum. The residue was dissolved in a mixture of dichloromethane (50 mL) and aqueous NaHCO3 (50 mL). The organic layer was dried and the solvent was removed to give the title compound 0305 (3.4 g, 83%). 1H NMR (DMSO-d6): delta 4.05 (s, 3H), 7.44 (s, 1H), 8.27 (s, 1H), 8.53 (s, 1H). |
| 83% | Step 22d. 4-Chloro-7-methoxy-6-nitroquinazoline (compound 0305); Compound 0304 (3.8 g, 17.2 mmol) was suspended in POCl3 (75 mL), the mixture was heated to reflux for 4 hours. The additional POCl3 was removed in a vacuum. The residue was dissolved in a mixture of dichloromethane (50 mL) and aqueous NaHCO3 (50 mL). The organic layer was dried and the solvent was removed to give the title compound 0305 (3.4 g, 83%). 1H NMR (DMSO-d6): delta 4.05 (s, 3H), 7.44 (s, 1H), 8.27 (s, 1H), 8.53 (s, 1H). |
| With thionyl chloride; In N,N-dimethyl-formamide; for 4h;Reflux; | DMF (0.5 mL) was added to a solution of 7-Methoxy-6-nitroquinazolin-4(3H)-one (1-g, 1.23 g, 5.56 mmol) in SOCl2 (8 mL) and the mixture was heated to reflux for 4 h. The volatiles were removed under reduced pressure to give (1-h, 1.2 g, 5.0 mmol). | |
| [054] DMF (0.5 mL) was added to a solution of 7-methoxy-6-nitroquinazolin-4(3H)-one (1-g, 1.23 g, 5.56 mmol) in SOCl2 (8 mL) and the mixture was heated to reflux for 4 h. The volatiles were removed under reduced pressure to give (1-h, 1.2 g, 5.0 mmol). | ||
| With thionyl chloride; In N,N-dimethyl-formamide; for 2h;Reflux; | A solution of 6-nitro-7-methoxy-3H-quinazolin-4-one (4.42 g, 20.0 mmol)Then 0.4 mL of DMF was added and the mixture was stirred at reflux for 2 h.The solution gradually turned brown,The reaction was stopped, cooled to room temperature,The excess S0C12 was distilled off,To give 4-chloro-6-nitro-7-methoxyquinazoline as a pale yellow solid. The resulting pale yellow solid was crushed and addedInto 30 mL of petroleum ether, the petroleum ether was distilled off under reduced pressure. The procedure was repeated twice with petroleum ether to remove the residual S0C12 to obtain a yellow solid.The yellow solid was transferred to a three-necked flask without purification and aniline (2.05 g, 22.0 mmol), isopropanol 170 mL, refluxStirred for 2 h, cooled to room temperature, the solid collected, washed with isopropanol, dried, yellow solid 6-nitro-7-methoxy-4-anilineYl) quinazoline in a yield of 61.9%. |
References: [1]Journal of Medicinal Chemistry,1996,vol. 39,p. 267 – 276.
[2]Patent: WO2008/33747,2008,A2 .Location in patent: Page/Page column 130.
[3]Patent: US2009/76022,2009,A1 .Location in patent: Page/Page column 74-75.
[4]Journal of Medicinal Chemistry,2010,vol. 53,p. 2000 – 2009.
[5]Patent: US2009/111772,2009,A1 .Location in patent: Page/Page column 73.
[6]Patent: WO2012/182,2012,A1 .Location in patent: Page/Page column 12.
[7]Patent: WO2012/356,2012,A1 .Location in patent: Page/Page column 12.
[8]Bulletin of the Korean Chemical Society,2015,vol. 36,p. 1933 – 1935.
[9]Journal of Medicinal Chemistry,2016,vol. 59,p. 8103 – 8124.
[10]Patent: CN103382182,2016,B .Location in patent: Paragraph 0272; 0273; 0280; 0281.
[2]Patent: WO2008/33747,2008,A2 .Location in patent: Page/Page column 130.
[3]Patent: US2009/76022,2009,A1 .Location in patent: Page/Page column 74-75.
[4]Journal of Medicinal Chemistry,2010,vol. 53,p. 2000 – 2009.
[5]Patent: US2009/111772,2009,A1 .Location in patent: Page/Page column 73.
[6]Patent: WO2012/182,2012,A1 .Location in patent: Page/Page column 12.
[7]Patent: WO2012/356,2012,A1 .Location in patent: Page/Page column 12.
[8]Bulletin of the Korean Chemical Society,2015,vol. 36,p. 1933 – 1935.
[9]Journal of Medicinal Chemistry,2016,vol. 59,p. 8103 – 8124.
[10]Patent: CN103382182,2016,B .Location in patent: Paragraph 0272; 0273; 0280; 0281.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 77% | With sodium; at 100℃; for 20h; | A mixture of compound 0303 (4.0 g,18.0 mmol) and sodium (2.4 g, 45 mmol) in methanol (50 mL) was heated at 100 0C in a sealed pressure vessel for 20 hours. The solution was neutralized with acetic acid and diluted with water to give the title compound 0304 (3.0 g, 77%). 1H NMR (DMSO-J6): £4.10. (s, 3H), 7.40 (s, IH), <n=”131″/>8.24 (s, IH), 8.50(s, IH), 12.67 (s, IH). |
| 77% | With sodium; at 100℃; for 20h; | Step 22c. 7-Methoxy-6-nitroquinazolin-4(3H)-one (compound 0304); A mixture of compound 0303 (4.0 g, 18.0 mmol) and sodium (2.4 g, 45 mmol) in methanol (50 mL) was heated at 100 C. in a sealed pressure vessel for 20 hours. The solution was neutralized with acetic acid and diluted with water to give the title compound 0304 (3.0 g, 77%). 1H NMR (DMSO-d6): delta4.10 (s, 3H), 7.40 (s, 1H), 8.24 (s, 1H), 8.50 (s, 1H), 12.67 (s, 1H). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 77% | With methanol; sodium; at 100℃; for 20h; | Step 22c. 7-Methoxy-6-nitroquinazolin-4(3H)-one (compound 0304) A mixture of compound 0303 (4.0 g, 18.0 mmol) and sodium (2.4 g, 45 mmol) in methanol (50 mL) was heated at 100 C. in a sealed pressure vessel for 20 hours. The solution was neutralized with acetic acid and diluted with water to give the title compound 0304 (3.0 g, 77%). 1H NMR (DMSO-d6): delta4.10 (s, 3H), 7.40 (s, 1H), 8.24 (s, 1H), 8.50 (s, 1H), 12.67 (s, 1H). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 87.6% | With potassium iodide; In N,N-dimethyl-formamide; at 90℃; for 20h; | A solution of 7-chloro-6-nitro-3H-quinazolin-4-one (7.01 g, 31.1mmol) in dry 120 mL DMF was added anhydrous sodium methoxide (5.458, 101.0mmol) ) and potassium iodide (5.168, 31.1mmol) and heated to 90 C for 20 h. The reaction solution suction filter, with acetic acid to adjust the filtrate to neutral, diluted with water, precipitation of solid, filtration, yellow6-nitro-7-methoxy-3H-quinazolin-4-one as a colorless solid, yield 87.6%. |










Reviews
There are no reviews yet.