Cat.NO.:A929545 Purity:95%
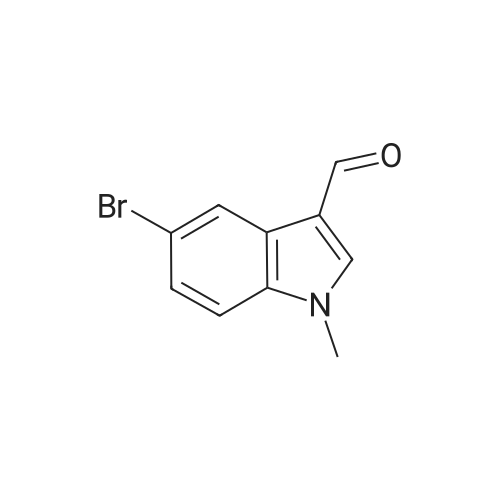
Product Details of [ 10102-94-0 ]
| CAS No. : | 10102-94-0 |
| Formula : |
C10H8BrNO |
| M.W : |
238.08
|
| SMILES Code : | O=CC1=CN(C)C2=C1C=C(Br)C=C2 |
| MDL No. : | MFCD03906373 |
| InChI Key : | NZJJLQUTQOICBN-UHFFFAOYSA-N |
| Pubchem ID : | 778451 |
Safety of [ 10102-94-0 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Computational Chemistry of [ 10102-94-0 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 13 |
| Num. arom. heavy atoms | 9 |
| Fraction Csp3 | 0.1 |
| Num. rotatable bonds | 1 |
| Num. H-bond acceptors | 1.0 |
| Num. H-bond donors | 0.0 |
| Molar Refractivity | 56.29 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
22.0 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
2.12 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
2.15 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
2.75 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
1.88 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
2.74 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
2.33 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-3.12 |
| Solubility | 0.182 mg/ml ; 0.000764 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-2.24 |
| Solubility | 1.36 mg/ml ; 0.0057 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-3.65 |
| Solubility | 0.0529 mg/ml ; 0.000222 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
Yes |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
Yes |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-6.23 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
0.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
1.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.34 |
Application In Synthesis of [ 10102-94-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 10102-94-0 ]
[ 10102-94-0 ] Synthesis Path-Downstream 1~35
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 90.1% | General procedure: 5-Fluoroindole-3-carbaldehyde (0.5 g, 3.07 mmol) and tetrahydrofuran (10 mL) were sequentially added to a 100 mL one-neck round bottom flask, cooled to 0 C, and slowly added NaH (60%, 196 mg, 4.91 mmol) After reacting for 10 minutes,Transfer to room temperature for 1 h, add MeI (0.38 mL, 6.14 mmol),The reaction was carried out at room temperature for 15.5 h, quenched with water (5 mL), and then evaporated to ethyl ether (ethyl acetate) (ethyl acetate (40mL×1)), washed with water (20mL×3), and then ethyl acetate was collected and then purified by column chromatography ( petroleum ether) /ethyl acetate (v/v) = 5/1) to give the title compound as a white solid(0.50 g, 92%). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | 5-bromoindole-3-carbaldehyde 2 (10 mmol) was dissolved in acetonitrile and to this powdered NaOH (5 mmol) was added and stirred for 10 min. Methyl iodide (10 mmol) was added dropwise to the reaction mixture. After 3h of stirring at room temperature, the solvent was completely evaporated. It was extracted with ethyl acetate (3×20 ml) and dried over Na2SO4. The combined organic layer was concentrated in vacuo to give a light yellow color solid purified by recrystallization with diethyl ether. Pale yellow solid; Yield: 96%; mp 122-124 C; IR (KBr) numax 3103, 2923, 2813, 1700, 1654. 1533, 1467, 1369, 1083, 799, 726; 1H NMR (DMSO-d6, 300 MHz) delta 9.88 (s, 1H), 8.33 (s, 1H), 8.23 (d, J = 1.70 Hz, 1H), 7.60 (d, J = 8.68 Hz, 1H), 7.48 (dd, J = 2.08, 8.87 Hz, 1H), 3.89 (s, 3H); TOF-HRMS (m/z) for C10H8BrNO, calculated 237.9862, observed 237.9855 [M+1] + | |
| 91% | With sodium hydride; In tetrahydrofuran; at 0℃; for 1.83333h; | 5-Bromo-1-methyl-lH-indole-3-carbaldehyde; 5-Bromo-1H-indole-3-carbaldehyde (4.8 mmol, 1076 mg) was dissolved in 15 mL of THF and the solution was cooled to 0 C under N2-athmosphere. Sodium hydride (11.7 mmol, 280 mg) was added carefully in portions and iodomethane (8.1 mmol, 1150 mg) was added. The mixture was stirred at 0 C for 1 h. More iodomethane (8.1 mmol, 1150 mg) was added and the stirring continued for 50 min. The mixture was poured over ice and the resulting slurry was extracted with EtOAc. The organic layer was dried over Na2SO4, filtered and evaporated. This gave 1037 mg (91 %) of the title product. ‘H NMR (400 MHz, MeOH-d4) 8 9.82 (s, 1H), 8,29 (m, 1H), 8.06 (s, 1H), 7.43 (m, 2H), 3.89 (s, 3H) |
| 81% | General procedure: To a suspension of NaH 60% in oil (2.25 equiv.) in dry DMF(0.8 mL/mmol) was added, at 0 C and under nitrogen atmosphere, a solution of indolecarboxaldehyde (1 equiv.) in dry DMF (2.5 mL/mmol). After stirring for 30 min at rt, alkyl halide (1.0e3.0 equiv.)was slowly added. After stirring overnight, the reaction wasquenched by addition of water and the product was extracted withdiethyl ether. The organic layer was dried over MgSO4, filtered offand concentrated under vacuum. The crude product was purifiedby column chromatography on silica gel. |
| General procedure: Compounds 7-9 were synthesized from the corresponding compounds 4-6. A solution of compounds 4-6 (60 mmol) in THF (30 mL) were added dropwise to a suspension of NaH (3.60 g, 60% dispersion in mineral oil, 150 mmol) in THF (30 mL) at 0 C. After stirring for 15 min, the heterogeneous mixture was treated with iodomethane (5.04 mL, 79.2 mmol) at room temperature for 1 h. Then the reaction mixture was cooled to 0 C, quenched with saturated NH4Cl (60 mL), and extracted with ether (3 * 50 mL). The organic layers were combined, washed with brine, dried over anhydrous Na2SO4 and concentrated in vacuo to give 1-Methyl-1H-indole-3-carboxaldehyde (7-9), a light brown solid. The crude 7-9 were used in the next step without any further purification. | ||
| With sodium hydride; In tetrahydrofuran; at 0 – 20℃; for 24h; | General procedure: The relevant indole-3-carboxaldehyde (2a-c, 10 mmol) in THF(25 mL) was added dropwise to a stirred solution of NaH (25 mmol)in THF (25 mL) at 0 C and CH3I (13.2 mmol) was added after 15 minstirring. The reaction mixturewas moved to room temperature andstirred for further 24 h. Then the solvent was removed in vacuumand the residue was extracted by ethyl acetate. The organic layerwas washed with brine, dried over anhydrous sodium sulfate,filtered and concentrated in vacuum. Recrystallization affordedcompound 3a-c with yields of 93.4-97.5%. |
[2]Journal of Medicinal Chemistry,2009,vol. 52,p. 6217 – 6223.
[3]European Journal of Medicinal Chemistry,2001,vol. 36,p. 545 – 553.
[4]Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,p. 3024 – 3028.
[5]Patent: WO2005/66132,2005,A1 .Location in patent: Page/Page column 47.
[6]European Journal of Medicinal Chemistry,2016,vol. 122,p. 408 – 418.
[7]Bioorganic and Medicinal Chemistry Letters,2007,vol. 17,p. 1793 – 1798.
[8]European Journal of Medicinal Chemistry,2015,vol. 99,p. 125 – 137.
[9]ChemMedChem,2016,vol. 11,p. 1446 – 1458.
[10]European Journal of Medicinal Chemistry,2018,vol. 156,p. 722 – 737.
[11]Organic Letters,2019,vol. 21,p. 2048 – 2051.
- 9
 [ 10102-94-0 ]
[ 10102-94-0 ]
 [ 7333-52-0 ]
[ 7333-52-0 ]
- 5-bromo-3-cyclopentylidenemethyl-1-methyl-1<i>H</i>-indole [ No CAS ]
[2]European Journal of Medicinal Chemistry,2001,vol. 36,p. 545 – 553.
[3]Chemical Biology and Drug Design,2011,vol. 77,p. 182 – 188.
[4]European Journal of Medicinal Chemistry,2015,vol. 99,p. 125 – 137.
[5]Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,p. 3024 – 3028.
[6]ChemMedChem,2016,vol. 11,p. 1446 – 1458.
[7]RSC Advances,2016,vol. 6,p. 30412 – 30424.
[8]European Journal of Medicinal Chemistry,2018,vol. 156,p. 722 – 737.
[9]Organic Letters,2019,vol. 21,p. 7702 – 7707.











Reviews
There are no reviews yet.