Cat.NO.:A118427 Purity:98%
Product Details of 4-Pyridinecarbonitrile
| CAS No. : | 100-48-1 |
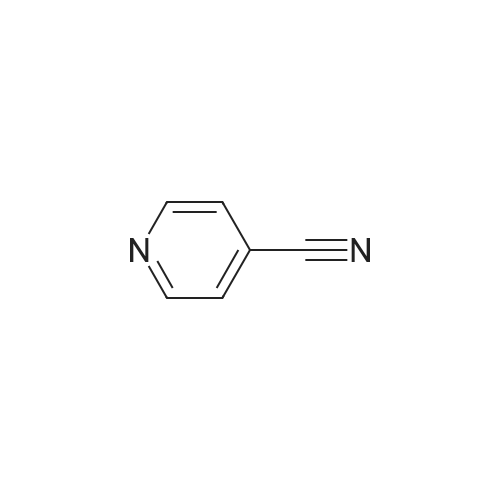
| Formula : |
C6H4N2 |
| M.W : |
104.11
|
| SMILES Code : | N#CC1=CC=NC=C1 |
| MDL No. : | MFCD00006417 |
| InChI Key : | GPHQHTOMRSGBNZ-UHFFFAOYSA-N |
| Pubchem ID : | 7506 |
Safety of 4-Pyridinecarbonitrile
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H312-H332 |
| Precautionary Statements: | P261-P264-P270-P271-P280-P301+P312+P330-P302+P352+P312-P304+P340+P312-P362+P364-P501 |
Application In Synthesis of 4-Pyridinecarbonitrile
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 100-48-1 ]
- Downstream synthetic route of [ 100-48-1 ]
[ 100-48-1 ] Synthesis Path-Upstream 1~8
[1] Chemistry – A European Journal, 2017, vol. 23, # 59, p. 14733 – 14737.
[1] Tetrahedron Letters, 2004, vol. 45, # 12, p. 2667 – 2669.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 71.9% | With ammonium peroxydisulfate; sulfuric acid; silver nitrate In dichloromethane; waterReflux | Step 1 : 2-Acetylisonicotinonitrile To a solution of isonicotinonitrile (52 g, 0.5 mol) in dichloromethane (1300 mL) and water (1 100 mL) were added ammonium persulfate ((NH4)2S208) (170 g, 0.75 mol), silver nitrate (6.8 g, 0.04 mol) and aqueous sulfuric acid (40 mL, 98percent sulfuric acid in 400 mL). A solution of 3-oxo-butyric acid (1 10 g, 1.25 mol) in dichloromethane (100 mL) was added dropwise while keeping the mixture refluxing. The reaction mixture was refluxed for 2 h. The resulting mixture was basified to pH -8-9 using sodium carbonate powder. The mixture was filtered and the filtrate was extracted with dichloromethane (500 mL x 3). The combined organics were dried over sodium sulfate, and concentrated under reduced pressure. The residue was recrystallized from ethanol to afford 2-acetylisonicotinonitrile (52.0 g, 71.9percent). |
[1] Patent: WO2013/150416, 2013, A1, . Location in patent: Page/Page column 117.
[1] Journal of Organic Chemistry, 1991, vol. 56, # 8, p. 2866 – 2869.
[1] Organic Letters, 2016, vol. 18, # 15, p. 3738 – 3741.
[1] Organic Letters, 2016, vol. 18, # 15, p. 3738 – 3741.
[1] Journal of Chemical Research, Miniprint, 1982, # 10, p. 2801 – 2815.
[2] Journal of Chemical Research, Miniprint, 1982, # 10, p. 2801 – 2815.
[1] Angewandte Chemie, 1985, vol. 97, # 8, p. 694 – 695.
[2] Heterocycles, 1987, vol. 26, # 3, p. 731 – 744.
[3] Angewandte Chemie, 1985, vol. 97, # 8, p. 694 – 695.
[4] Angewandte Chemie, 1985, vol. 97, # 8, p. 694 – 695.
[5] Heterocycles, 1987, vol. 26, # 3, p. 731 – 744.
[6] Heterocycles, 1987, vol. 26, # 3, p. 731 – 744.




























Reviews
There are no reviews yet.