Cat.NO.:A192561 Purity:95%
Product Details of [ 100114-58-7 ]
| CAS No. : | 100114-58-7 |
| Formula : |
C6H8N2O |
| M.W : |
124.14
|
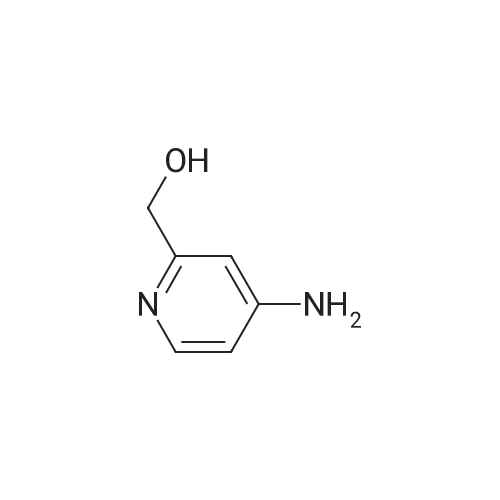
| SMILES Code : | OCC1=NC=CC(N)=C1 |
| MDL No. : | MFCD11100581 |
| InChI Key : | ZBKZWFGHOALGNP-UHFFFAOYSA-N |
| Pubchem ID : | 23564158 |
Safety of [ 100114-58-7 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Computational Chemistry of [ 100114-58-7 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 9 |
| Num. arom. heavy atoms | 6 |
| Fraction Csp3 | 0.17 |
| Num. rotatable bonds | 1 |
| Num. H-bond acceptors | 2.0 |
| Num. H-bond donors | 2.0 |
| Molar Refractivity | 34.77 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
59.14 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
0.75 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
-0.68 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
0.01 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
-0.75 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
0.49 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
-0.04 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-0.61 |
| Solubility | 30.6 mg/ml ; 0.246 mol/l |
| Class?
Solubility class: Log S scale |
Very soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-0.09 |
| Solubility | 101.0 mg/ml ; 0.818 mol/l |
| Class?
Solubility class: Log S scale |
Very soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-1.45 |
| Solubility | 4.39 mg/ml ; 0.0354 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
No |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-7.54 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
1.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.33 |
Application In Synthesis of [ 100114-58-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100114-58-7 ]
[ 100114-58-7 ] Synthesis Path-Downstream 1~6
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With ethanol; potassium hydroxide; for 2.0h;Reflux; | Step 2. (4-Amino-2-pyridyl)methanol: N-[2-(Hydroxymethyl)-4-pyridyl]acetamide (0.050 g, 0.30 mmol) was dissolved in EtOH and treated with KOH (0.033 g, 0.60 mmol). The solution was heated at reflux for 2 h. The solution was concentrated under a stream of nitrogen gas and the residue dissolved with DCM. The organic solution was dried (Na2S04) and concentrated to give the title compound. 1H NMR (DMSO-d6) delta 7.86 (d, 1H), 6.60 (d, 1H), 6.30 (dd, 1H), 5.97 (s, 2H), 5.22 (s, 1H), 4.34 (s, 2H). |
- 2
 [ 1918-02-1 ]
[ 1918-02-1 ]
 [ 100114-58-7 ]
[ 100114-58-7 ]
 [ 100047-36-7 ]
[ 100047-36-7 ]
- 4-amino-3-chloro-pyridine-2-carboxylic acid [ No CAS ]

- 4-amino-6-chloropyridine-2-carboxylic acid [ No CAS ]
- 5
 [ 100114-58-7 ]
[ 100114-58-7 ]
- methyl 3-chloro-1-methyl-4-[[(1R)-2,2,2-trifluoro-1-methyl-ethyl]sulfamoyl]pyrrole-2-carboxylate [ No CAS ]

- 3-chloro-N-[2-(hydroxymethyl)-4-pyridyl]-1-methyl-4-[[(1R)-2,2,2-trifluoro-1-methylethyl]sulfamoyl]pyrrole-2-carboxamide [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 249 mg | With lithium hexamethyldisilazane; In tetrahydrofuran; at 20.0℃; | Methyl 3-chloro-l-methyl-4-[[(lR)-2,2,2-trifluoro-l-methyl-ethyl]sulfamoyl]pyrrole-2- carboxylate (400 mg, 0.57 mmol) and <strong>[100114-58-7](4-aminopyridin-2-yl)methanol</strong> (157 mg, 1.26 mmol) were dissolved in dry THF (5 mL). Lithium bis(trimethylsilyl)amide (1M in THF) (3.4 mL, 1 M, 3.4 mmol) was added drop wise and the reaction mixture was stirred overnight at room temperature. The reaction mixture was next quenched with sat. NH4C1 (10 mL). The organic layer was removed and the aqueous layer extracted with CH2CI2 (2 X 5 mL). The combined organic layers were evaporated to dryness and the residue was purified on silica using a heptane to EtOAc gradient yielding 3-chloro-N-[2-(hydroxymethyl)-4-pyridyl]-l-methyl-4-[[(lR)-2,2,2- trifluoro-l-methyl-ethyl]sulfamoyl]pyrrole-2-carboxamide (249 mg) as an off-white powder after trituration with diisopropylether. Method B: Rt: 0.81 min. m/z: 439 (M-H)” Exact mass: 440.1. 1H NMR (400 MHz, DMSO-d6) 5 ppm 1.19 (d, J=7.0 Hz, 3 H), 3.78 (s, 3 H), 3.92 – 4.05 (m, 1 H), 4.53 (d, J=5.7 Hz, 2 H), 5.42 (t, J=5.8 Hz, 1 H), 7.55 (dd, J=5.5, 2.0 Hz, 1 H), 7.68 (s, 1 H), 7.79 (d, J=1.5 Hz, 1 H), 8.38 (d, J=5.5 Hz, 1 H), 8.50 (br. s., 1 H), 10.69 (s, 1 H). DSC: From 30 to 300 C at 10C/min, peak 233.9 C. 3-chloro-N-[2-(hydroxymethyl)-4-pyridyl]-l- methyl-4-[[(lR)-2,2,2-trifluoro-l-methyl-ethyl]sulfamoyl]pyrrole-2-carboxamide (181 mg, 0.41 mmol) was dissolved in THF (5 mL). (Diethylamino)sulfur trifluoride (108.5 mu, 0.82 mmol) was added and the reaction mixture was stirred overnight at room temperature. The volatiles were removed under reduced pressure and the residue was purified via prep. HPLC (Stationary phase: RP XBridge Prep C18 OBD-IotaOmicronmuiotaeta, 30x150mm, Mobile phase: 0.25% (0700) NH4HCO3 solution in water, MeOH) yielding 3-chloro-N-[2-(fluoromethyl)-4-pyridyl]-l- methyl-4-[[(lR)-2,2,2-trifluoro-l-methyl-ethyl]sulfamoyl]pyrrole-2-carboxamide (11.2 mg). Method B: Rt: 0.97 min. m/z: 441.1 (M-H)” Exact mass: 442.0. 1H NMR (400 MHz, (0701) CHLOROFORM-d) delta ppm 1.39 (d, J=6.8 Hz, 3 H), 3.93 – 3.99 (m, 1 H), 4.00 (s, 3 H), 5.49 (d, J=46.9 Hz, 2 H), 7.37 (s, 1 H), 7.58 – 7.64 (m, 2 H), 8.52 (d, J=5.3 Hz, 1 H). |
- 6
 [ 67-56-1 ]
[ 67-56-1 ]
 [ 100114-58-7 ]
[ 100114-58-7 ]
- nickel dichloride [ No CAS ]

- [Ni4(2-(hydroxymethyl)-4-aminopyridine)4Cl4(MeOH)4] [ No CAS ]



Reviews
There are no reviews yet.