Cat.NO.:A189963 Purity:95%
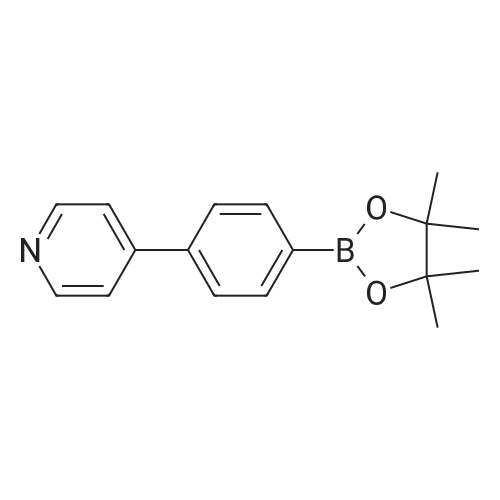
Product Details of [ 1009033-87-7 ]
| CAS No. : | 1009033-87-7 |
| Formula : |
C17H20BNO2 |
| M.W : |
281.16
|
| SMILES Code : | CC1(C)OB(OC1(C)C)C1=CC=C(C=C1)C1=CC=NC=C1 |
| MDL No. : | MFCD12828171 |
| InChI Key : | PTNMCYWJKRZCDE-UHFFFAOYSA-N |
| Pubchem ID : | 58526497 |
Safety of [ 1009033-87-7 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H332-H335 |
| Precautionary Statements: | P261-P280-P305+P351+P338 |
Computational Chemistry of [ 1009033-87-7 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 21 |
| Num. arom. heavy atoms | 12 |
| Fraction Csp3 | 0.35 |
| Num. rotatable bonds | 2 |
| Num. H-bond acceptors | 3.0 |
| Num. H-bond donors | 0.0 |
| Molar Refractivity | 86.15 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
31.35 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
0.0 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
3.46 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
3.05 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
1.89 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
2.9 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
2.26 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-4.05 |
| Solubility | 0.0248 mg/ml ; 0.0000883 mol/l |
| Class?
Solubility class: Log S scale |
Moderately soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-3.8 |
| Solubility | 0.0446 mg/ml ; 0.000158 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-6.14 |
| Solubility | 0.000205 mg/ml ; 0.000000728 mol/l |
| Class?
Solubility class: Log S scale |
Poorly soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
Yes |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
Yes |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
Yes |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
Yes |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-5.56 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
0.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
1.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<0.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
2.87 |
Application In Synthesis of [ 1009033-87-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1009033-87-7 ]
[ 1009033-87-7 ] Synthesis Path-Downstream 1~30
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 100% | A 100 mL sealed tube was charged with 4-(4-bromophenyl)pyridine (0.9 g, 3.8mmol), bis(pinacolato)diboron (1.17 g, 4.61 mmol), potassium acetate (0.745 g, 7.6mmol) and 1-4 dioxane (10 mL). The reaction mixture was purged with argon for 30mm. Then, Pd(dppf)C12 (0.75 g, 0.05 eq) was added and heated at 100 00 over night.After cooing, the reaction mixture was extracted with ethyl acetate. The organic layer was washed with water and dried over anhydrous Na2SO4. The organic layer was concentrated under vacuo to yield crude product, which was purified by combif lash to yield title compound (1.0 g, 100.0percent). LCMS: (M+H) = 282.1 |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 61% | With caesium carbonate;palladium diacetate; XPhos; In tetrahydrofuran; for 22h;Inert atmosphere; Reflux; | In a stream of argon, 4-[4-(4,4,5,5-tetramethyl-1,3,2-dioxabororan-2-yl)phenyl]pyridine (420 mg), 2,4-bis(5-chlrobiphenyl-3-yl)-6-phenyl-1,3,5-triazine (263 mg), cesium carbonate (485 mg), palladium acetate (5.6 mg) and 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (23.7 mg) were suspended in tetrahydrofuran (15 mL), and the suspension was refluxed for 22 hours. The reaction mixture was left to stand at room temperature, and then low-boiling ingredients were distilled away under a reduced pressure. Methanol was added to the obtained solid. The solid precipitate was recovered by filtration, and the obtained crude product was purified by silica gel chromatography using a methanol/chloroform (1:100 – 1:50) mixed liquid as a developing solvent to give 232 mg of the target 6-phenyl-2,4-bis[4-(4-pyridyl)-1,1′:3′,1′-terphenyl-5′-yl]-1,3,5-triazine as a white powder (yield: 61%). 1H-NMR (CDCl3) :delta7.49(brt,J=7.1Hz,2H) 7.57(d,J=7.5Hz, 4H), 7.61-7.66(m,7H),7.82-7.86(m,8H),7.94(d,J=8.5Hz,4H),8.10(t,J= 1.7Hz,2H),8.73(dd,J=4.5,1.6Hz,4H),8.84(brdd,J=7.7,1.7Hz,2H), 9.04(d,J=1.7Hz,4H). 13C-NMR(CDCl3) :delta121.5(CH*4),126.5(CH*2) ,127.1(CH*2) ,127.4 (CH*4),127.6(CH*4),127.9(CH*2),128.1(CH*4),128.7(CH*2),129.0 (CH*4),129.1(CH*2),130.0(CH*2),132.8(CH),136.1(quart.),137.5 (quart.*2),137.6(quart.*2),140.7(quart.*2),141.4(quart.*2), 141.6(quart.*2),142.6(quart.*2),147.8(quart.*2),150.4(CH*4), 171.8(quart.*2),171.9(quart.). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 58% | With caesium carbonate; XPhos;palladium diacetate; In tetrahydrofuran; for 87h;Inert atmosphere; Reflux; | In a stream of argon, 0.40 g (0.81 mmol) of 2-(3,5-dibromophenyl)-4,6-di-p-tolylpyrimidine, 0.48 g (1.70 mmol) of 4-[4-(4,4,5,5-tetramethyl-1,3,2-dioxabororan-2-yl)phenyl]-pyridine, 0.55 g (1.70 mmol) of cesium carbonate, 7 mg (0.032 mmol) of palladium acetate and 31 mg (0.065 mmol) of 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl were suspended in 20 mL of tetrahydrofuran, and the obtained suspension was heated under reflux for 87 hours. The reaction mixture was cooled to room temperature, and was then distilled under a reduced pressure to remove all volatile materials. Methanol was added to the concentrate and the thus-deposited solid was collected by filtration. The thus-obtained crude product was purified by silica gel chromatography using a chloroform/methanol (100:1) mixed solvent as an eluent to give 0.30 g of the target 2-[4,4″-di(4-pyridyl)-1,1′ :3′,1″-terphenyl- 5′-yl]-4,6-di-p-tolylpyrimidine as a white solid (yield: 58%). 1H-NMR(CDCl3):delta2.50(s,6H),7.41(d,J=8.0Hz,4H),7.63(dd,J=4.5, 1.5Hz,4H),7.84(d,J=8.3Hz,4H),7.95(d,J=8.3Hz,4H),8.03(t,J=1.8Hz, 1H),8.05(s,1H),8.24(d,J=8.0Hz,4H),8.73(dd,J=4.5,1.5Hz,4H),9.02(d, J=1.8Hz,2H). 13C-NMR(CDCl3):delta21.6(CH3),31.0(CH3),110.1(CH),121.5(CH×4), 126.7(CH×2),127.3(CH×4),127.5(CH×4),128.2(CH×4),129.7(CH×4),134.7 (quart.×2),137.3(quart.×2),139.9(quart.),141.2(quart.×2),141.3 (quart.×2),142.0(quart.×2),147.9(quart.×2),150.4(CH×4), 164.0(quart.),164.7(quart.×2). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 90% | With potassium carbonate;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; water; at 90℃; for 12h; | 15.0 g (32.2 mmol) of the intermediate product (N), 10.9 g (38.6 mmol) of <strong>[1009033-87-7]4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine</strong>, and 1.1 g (1.0 mmol) of tetrakis(triphenylphosphine)palladium [Pd(PPh3)4] were dissolved in 300 mL of a tetrahydrofuran (THF) solvent. A solution in which 8.9 g (64.4 mmol) of potassium carbonate (K2CO3) was dissolved in 100 ml of water was added thereto, and they were reacted at 90 C. for 12 hours. The solvent was removed under a reduced pressure, and the reaction product was rinsed with water and methanol. The residues were recrystallized with toluene, precipitated crystals were separated by a filter, rinsed with toluene, and dried to provide a white solid of a compound in 17.0 g (yield: 90%). (calculation value: 584.71, measurement value: MS[M+1] 585.01) |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 64% | With potassium carbonate;tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; water; at 90℃; for 12h; | 20.0 g (23.6 mmol) of the intermediate product (N), 18.1 g (64.4 mmol) of <strong>[1009033-87-7]4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine</strong>, and 1.5 g (1.3 mmol) of tetrakis(triphenylphosphine)palladium [Pd(PPh3)4] were dissolved in 400 ml of a tetrahydrofuran (THF) solvent. A solution in which 11.9 g (85.8 mmol) of potassium carbonate (K2CO3) was dissolved in 200 ml of water was added thereto, and then they were reacted at 90 C. for 12 hours. The solvent was removed under a reduced pressure, and the reaction product was rinsed with water and methanol. The residues were recrystallized with toluene, precipitated crystals were separated by a filter, rinsed with toluene, and dried to provide a white solid of a compound in 16.0 g (yield: 64%). (calculation value: 584.71, measurement value: MS[M+1] 585.01) |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| General procedure: Step-ii: 1-(2-fluorobenzyl)-4-( 4,4,5 ,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1 H-pyrazole1-(2-fluorobenzyl)-4-iodo-1H-pyrazole (2.25 g, 7.4 mmol) and bispinocalatodiboron (2.07 g,8.2 mmol) were added to a solution of DMSO (20 ml) previously purged with argon (10min). The reaction mixture was purged with argon for a further 15mins, followed by the10 addition of potassium acetate (2.19 g, 22.3 mmol) andbis(triphenylphosphine)palladium(II)dichloride (261 mg, 0.3725 mmol). The resultingmixture was heated to reflux at 80 oc overnight. The reaction was monitored by TLC (40%ethyl acetate in hexane). The reaction mixture was cooled and diluted with ethyl acetate (100ml) and filtered over celite bed and the filtrate was washed with cold water (2×100 ml). The15 organic layer was dried over NazS04, and concentrated under reduced pressure to afford 2.3 gof the crude product which was taken as such for next reaction. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 70.5% | Using similar reaction conditions as described in step-ii of example-1, 5-bromo-3-(1-(3-5 fluorobenzyl)-3,5-dimethyl-1 H-pyrazol-4-yl)-l-tosyl-1H-pyrrolo[2,3-b] pyridine (step-i ofexample-14) (150mg, 0.27mmol) was coupled with <strong>[1009033-87-7]4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine</strong> (intermediate 45) (91.44mh, 0.32mmol) in sodiumcarbonate (85.86, 0.81mmol), toluene/ethanol/water (5/5/2ml). This afforded 120mg (70.5%yield) of the titled compound. |











Reviews
There are no reviews yet.