Cat.NO.:A895756 Purity:98%
Product Details of [ 1011-17-2 ]
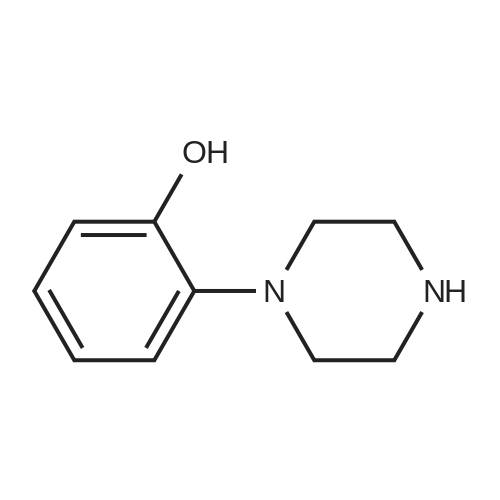
| CAS No. : | 1011-17-2 |
| Formula : |
C10H14N2O |
| M.W : |
178.23
|
| SMILES Code : | OC1=CC=CC=C1N2CCNCC2 |
| MDL No. : | MFCD00190246 |
| InChI Key : | UORNTHBBLYBAJJ-UHFFFAOYSA-N |
| Pubchem ID : | 70530 |
Safety of [ 1011-17-2 ]
| GHS Pictogram: |   |
| Signal Word: | Danger |
| Hazard Statements: | H314-H302+H312 |
| Precautionary Statements: | P264-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P403+P233-P501 |
| Class: | 8 |
| UN#: | 3259 |
| Packing Group: | Ⅲ |
Application In Synthesis of [ 1011-17-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1011-17-2 ]
[ 1011-17-2 ] Synthesis Path-Downstream 1~2
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 84% | With potassium carbonate; potassium iodide; In acetonitrile;Reflux; Inert atmosphere; | General procedure: A mixture of <strong>[129722-34-5]7-(4-bromobutoxy)-3,4-dihydroquinolin-2(1H)-one</strong> (20, 1.7 mmol), 4-(chlorophenyl)-4-hydroxypiperidine (1.7 mmol), potassium carbonate (5 equiv) and potassium iodide (1 equiv) in acetonitrile (20 mL) was stirred at reflux overnight. The reaction mixture was evaporated. The resulting residue was suspended in water (25 mL) and filtered. The solid was washed with water and air dried to give the product as white powder (96percent yield). The oxalate salt was prepared using 1 equiv of oxalic acid in ethanol and recrystallized from ethanol to give 2 as an off-white powder. |







Reviews
There are no reviews yet.