Cat.NO.:A1149351 Purity:98%
Product Details of [ 10124-78-4 ]
| CAS No. : | 10124-78-4 |
| Formula : |
C11H7N |
| M.W : |
153.18
|
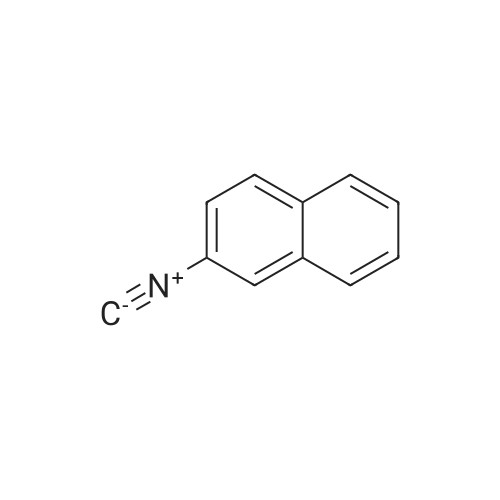
| SMILES Code : | [C-]#[N+]C1=CC=C2C=CC=CC2=C1 |
| MDL No. : | MFCD06200692 |
| InChI Key : | ZZKQDMRCHFQQCQ-UHFFFAOYSA-N |
| Pubchem ID : | 16217475 |
Safety of [ 10124-78-4 ]
| GHS Pictogram: |  |
| Signal Word: | Danger |
| Hazard Statements: | H301-H311-H315-H319-H331-H335 |
| Precautionary Statements: | P261-P264-P270-P271-P280-P302+P352-P304+P340-P305+P351+P338-P310-P330-P332+P313-P337+P313-P361-P403+P233-P405-P501 |
| Class: | 6.1 |
| UN#: | 2811 |
| Packing Group: | Ⅲ |
Application In Synthesis of [ 10124-78-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 10124-78-4 ]
[ 10124-78-4 ] Synthesis Path-Downstream 1~3
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With acetic acid; In trifluoroethanol; for 2h; | Step A: Methyl-6-[1-(4-methylpiperazin-1-yl)-2-(2-naphthylamino)-2-oxoethyl]nicotinate. Methyl 6-formylnicotinate (50.0 mg, 0.303 mmol), acetic acid (19.0 muL, 0.333 mmol), N-methylpiperizine (40.4 muL, 0.364 mmol), and 2-napthylisocyanide (51.0 mg, 0.333 mmol) were dissolved in 100 muL of TFE. The solution was allowed to stir for 2 h then purified by reverse phase HPLC to give the desired product MS cal’d 419 (MH+), exp 419 (MH+). |
- 3
 [ 53137-27-2 ]
[ 53137-27-2 ]
 [ 10124-78-4 ]
[ 10124-78-4 ]
 [ 100-52-7 ]
[ 100-52-7 ]
 [ 106-49-0 ]
[ 106-49-0 ]
- 2,4-dimethyl-N-(2-(naphthalen-1-ylamino)-2-oxo-1-phenylethyl)-N-(p-tolyl)thiazole-5-carboxamide [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 18% | General procedure: The aldehyde (0.8 equivalent) and amine (0.7 equivalent) were dissolved in methanol (2.0 mL) and stirred for two to 3 h depending upon the starting material. The acid (100 mg, 1 equivalent) and isocyanide (0.7 equivalent) were added in the reaction mixture and further stirred. The reaction mixture was monitored using TLC analysis.Water (4 mL) was added upon completion of the reaction.The resulted solid was filtered off and dissolved in ethyl acetate(10 mL), washed with water (2 3 mL) and dried over sodium sulphate. The crude product was purified using silica gel column chromatography. The ethyl acetate:hexane (6:4) solvent system was used for the purification of these compounds. |






Reviews
There are no reviews yet.