Cat.NO.:A134853 Purity:97%
Product Details of [ 1003709-47-4 ]
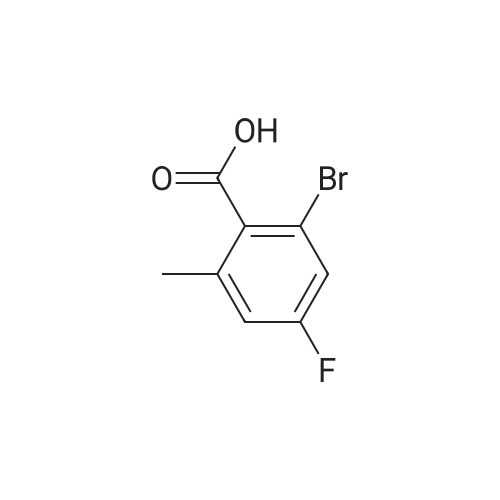
| CAS No. : | 1003709-47-4 |
| Formula : |
C8H6BrFO2 |
| M.W : |
233.03
|
| SMILES Code : | CC1=CC(F)=CC(Br)=C1C(O)=O |
| MDL No. : | MFCD09263435 |
| Boiling Point : | No data available |
| InChI Key : | VOFBSAUUUDKWGR-UHFFFAOYSA-N |
| Pubchem ID : | 45073547 |
Safety of [ 1003709-47-4 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of [ 1003709-47-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 1003709-47-4 ]
[ 1003709-47-4 ] Synthesis Path-Upstream 1~1
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 100% | With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 23 h; | Step eMethyl 2-bromo-4-fluoro-6-methylbenzoateA mixture of 2-bromo-4-fluoro-6-methylbenzoic acid (1.94 g, 8.33 mmol), anhydrous potassium carbonate (1.72 g,12.5 mmol), iodomethane (2.36 g, 17 mmol) in N,N-dimethylformamide (15 mL) was vigorously stirred for 23 hours at20 0C. The suspension was poured into 70 mL of water. A heavy liquid separated. The product was extracted with ethyl acetate (4 x 25 mL). The organic phase was washed with water (5 x 20 mL), brine (2 x 20 mL), dried with sodium sulfate, filtered and concentrated to give 2.07 g (100 percent) of pure ester. 1H NMR (400 MHz, CDCI3) δ ppm 7.18(dd, 3JH-F = 8.1 Hz, 4JH-H = 2.4 Hz, 1H, Ar), 6.91 (dd, 3JH-F = 9.0 Hz, 4JH-H = 2.2 Hz, 1H, Ar), 3.96 (s, 3H, OCH3), 2.35(s, 3H, CH3). |
| 100% | With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 23 h; | A mixture of 2-bromo-4-fluoro-6-methyl-benzoic acid (VII) (1.94 g, 8.33 mmol), anhydrous potassium carbonate (1.72g, 12.5 mmol), methyl iodide (2.36 g, 17 mmol) in N,N-dimethylformamide (15 mL) was vigorously stirred for 23 h at20 00 The suspension was poured into 70 mL of water. A dense oil separated out. The product was extracted with ethyl acetate (4 x 25 mL). The organic phase was washed with water (5 x 20 mL), brine (2 x 20 mL), dried over Na2504, filtered and concentrated to give 2.07g (quantitative yield) of 2-bromo-4-fluoro-6-methyl-benzoic acid methyl ester (VI).1H NMR (400.5 MHz, ODd3) ppm 2.35 (s, 3H), 3.96 (s, 3H), 6.91 (dd, JH-F = 9.0 Hz, JH-H = 2.2 Hz, 1H), 7.18 (dd, JHF = 8.1 Hz, JH-H = 2.4 Hz, 1H). |
References:
[1] Patent: WO2011/6803, 2011, A1, . Location in patent: Page/Page column 35-36.
[2] Patent: WO2014/64149, 2014, A1, . Location in patent: Page/Page column 27; 28.







Reviews
There are no reviews yet.