Cat.NO.:A110938 Purity:95%
Product Details of 1H-Imidazole-2-carbaldehyde
| CAS No. : | 10111-08-7 |
| Formula : |
C4H4N2O |
| M.W : |
96.09
|
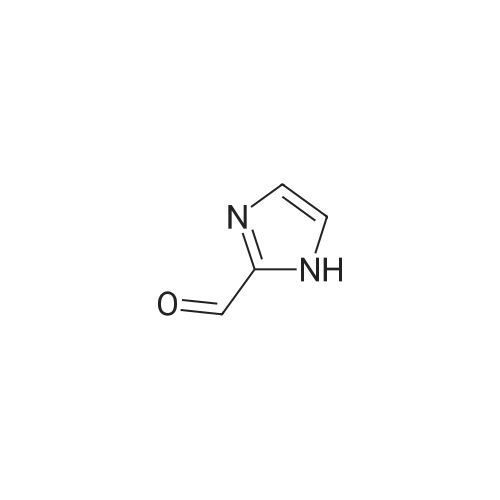
| SMILES Code : | O=CC1=NC=CN1 |
| Synonyms : |
2-Formylimidazole
|
| MDL No. : | MFCD00003544 |
| InChI Key : | XYHKNCXZYYTLRG-UHFFFAOYSA-N |
| Pubchem ID : | 24955 |
Safety of 1H-Imidazole-2-carbaldehyde
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of 1H-Imidazole-2-carbaldehyde
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 10111-08-7 ]
- Downstream synthetic route of [ 10111-08-7 ]
[ 10111-08-7 ] Synthesis Path-Upstream 1~1
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 78% | at 5 – 20℃; for 3 h; | To a solution of compound 1 H-imidazole-2-carbaldehyde (5g, 52.08 mmol) in MeOH (50 mL) was added sodium borohydride (3.93g, 104.16 mmol) portion-wise, at 5 00, and the reaction mixture was allowed to stir at RT for 3h. The reaction mixture was quenched with brine (25 mL) and concentrated in vacuo. The crude compound was purified by silica gel column chromatography eluting with 10percent MeOH/CHCI3) to obtain (1H-imidazol-2-yl)-methanol as a pale yellow solid (4g, 78percent).R:0.1 (10percent MeOH/CHCI3).1H NMR (400MHz, CD3OD): O 6.97 (5, 2H), 4.61 (5, 2H). |
| 51% | With sodium tetrahydroborate In methanol at 20℃; for 1 h; Inert atmosphere | To a solution of 2-imidazolecarboxyaldehyde (18-1) (1.92 g, 20 mmol, 1.0 eq.) was suspended in methanol (30 ml), NaBH4 (1.52g, 40 mmol, 2.0 eq.) was added portion-wise. The reaction mixture was stirred at room temperature for 1 h under N2. It was quenched with 5 ml of brine. The solvent was removed and the solid was purified with silica gel column chromatography (DCM : MeOH = 20: 1) to afford 18-2 as a white solid. (1.0g, Yield: 51percent). |
| 51% | at 20℃; for 1 h; Inert atmosphere | Compound 26-2 (0449) To a solution of 2-imidazolecarboxyaldehyde (26-1) (1.92 g, 20 mmol, 1.0 eq) was suspended in methanol (30 mL), NaBH4 (1.52 g, 40 mmol, 2.0 eq) was added portion-wise. The reaction mixture was stirred at room temperature for 1 h under N2. It was quenched with 5 mL of brine. The solvent was removed and the solid was purified with silica gel column chromatography (DCM:MeOH=20:1) to afford a white solid. (1.0 g, Yield: 51percent). |
| 45.2% | With sodium tetrahydroborate In methanol; dichloromethane | Part A Preparation 2-hydroxymethyl-1H-imidazole 2-Imidazolecarboxyaldehyde (5.0 g, 52.0 mmol) was suspended in 200 mL of methanol. NaBH4 (3.95 g, 0.10 mol) was added portion-wise. The reaction mixture was stirred at room temperature for 1 h under N2. It was quenched with 10 mL of brine. The solvent was removed. The solid was washed with 5percent MeOH in CH2Cl2. The inorganic solid was filtered off. The filtrate was concentrated and chromatographed with 5percent MeOH in CH2Cl2 to give 2.32 g off-white solid (45.2percent yield). 1H NMR (DMSO-d6): δ 6.86 (s, 2H), 4.40 (s, 2H). |
[1] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 3, p. 827 – 833.
[2] Patent: WO2015/36759, 2015, A1, . Location in patent: Page/Page column 200.
[3] Journal of Organic Chemistry, 2010, vol. 75, # 10, p. 3208 – 3213.
[4] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 19, p. 5849 – 5853.
[5] Patent: US9138427, 2015, B2, . Location in patent: Page/Page column 302.
[6] Journal of Medicinal Chemistry, 2005, vol. 48, # 6, p. 1729 – 1744.
[7] Patent: US2003/144287, 2003, A1, .
[8] Biological and Pharmaceutical Bulletin, 1998, vol. 21, # 9, p. 958 – 963.
[9] Journal of Medicinal Chemistry, 2004, vol. 47, # 15, p. 3707 – 3709.















Reviews
There are no reviews yet.