Cat.NO.:A639618 Purity:98%
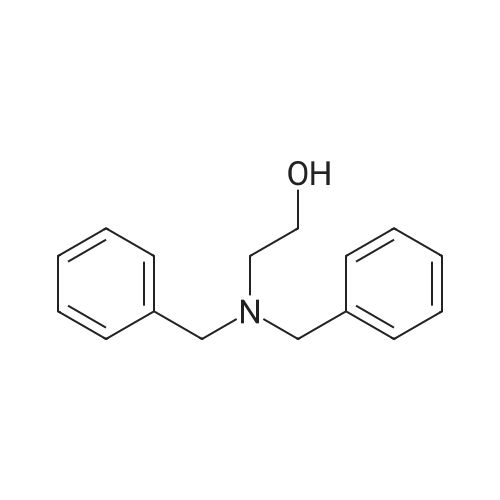
Product Details of [ 101-06-4 ]
| CAS No. : | 101-06-4 |
| Formula : |
C16H19NO |
| M.W : |
241.33
|
| SMILES Code : | OCCN(CC1=CC=CC=C1)CC1=CC=CC=C1 |
| MDL No. : | MFCD00020574 |
| InChI Key : | WTTWSMJHJFNCQB-UHFFFAOYSA-N |
| Pubchem ID : | 22657 |
Safety of [ 101-06-4 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Computational Chemistry of [ 101-06-4 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 18 |
| Num. arom. heavy atoms | 12 |
| Fraction Csp3 | 0.25 |
| Num. rotatable bonds | 6 |
| Num. H-bond acceptors | 2.0 |
| Num. H-bond donors | 1.0 |
| Molar Refractivity | 74.37 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
23.47 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
2.76 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
2.57 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
2.38 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
2.92 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
3.1 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
2.75 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-3.05 |
| Solubility | 0.214 mg/ml ; 0.000886 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-2.71 |
| Solubility | 0.47 mg/ml ; 0.00195 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-5.3 |
| Solubility | 0.00121 mg/ml ; 0.000005 mol/l |
| Class?
Solubility class: Log S scale |
Moderately soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
Yes |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
Yes |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-5.95 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
0.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.27 |
Application In Synthesis of [ 101-06-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 101-06-4 ]
[ 101-06-4 ] Synthesis Path-Downstream 1~35
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| In methanol; | A. Condensed ethylene oxide (7 ml, 125 mmol) was added to a mixture of dibenzylamine (19.2 ml, 100 mmol) in methanol (20 ml) cooled to 0C. After 2 hours at room temperature, the mixture was evaporated without heating above room temperature. The residue was distilled to afford the title compound, bp. 220-225C/25 mm, which solidified on standing, mp 48C. | |
| at 130℃; under 3102.97 Torr; for 18h;Inert atmosphere; Autoclave; Large scale; | Dibenzylamine (7627 g) was charged to a 24-L N2-purged pressure vessel. The reactor was pressurized and vented several times with N2, then pressurized with N2 and heated to 130 C. Ethylene oxide (1880 g) was fed into the reactor at such a rate to keep the pressure below 60 psia (?4 h). Upon completing the ethylene oxide addition, the reaction mixture was stirred at 130 C for 14 h. After lowering the temperature to 60 C and releasing pressure, 45%potassium hydroxide (70 g) was added to the vessel and the reaction mixture stirred at 110 C to remove water. The temperature was increased to 130 C and butylene oxide (11,277 g) was fed into the reactor at such a rate to keep the pressure below 60 psia (~31 h). Upon completing the addition, the reaction mixture was stirred for 4 h at 130 C. The reaction mixture was cooled to room temperature and the reaction mixture neutralized with acetic acid. |
- 5
 [ 2199-94-2 ]
[ 2199-94-2 ]
 [ 101-06-4 ]
[ 101-06-4 ]
- 6-bromo-2-oxo-2<i>H</i>-chromene-3-carboxylic acid-(2-dibenzylamino-ethyl ester) [ No CAS ]
- 6
 [ 1729-01-7 ]
[ 1729-01-7 ]
 [ 101-06-4 ]
[ 101-06-4 ]
- 8-methoxy-2-oxo-2<i>H</i>-chromene-3-carboxylic acid-(2-dibenzylamino-ethyl ester) [ No CAS ]
- 7
 [ 3855-87-6 ]
[ 3855-87-6 ]
 [ 101-06-4 ]
[ 101-06-4 ]
- 6,8-dibromo-2-oxo-2<i>H</i>-chromene-3-carboxylic acid-(2-dibenzylamino-ethyl ester) [ No CAS ]
[2]Journal of the Chemical Society,1949,p. 500,505.
[3]Canadian Journal of Chemistry,1965,vol. 43,p. 3119 – 3128.
[4]European Journal of Organic Chemistry,2014,vol. 2014,p. 1103 – 1109.
[5]Patent: DE538456,1930, .
Fortschr. Teerfarbenfabr. Verw. Industriezweige,vol. 18,p. 2980.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With dibromo sulfoxide; In cyclohexane; N,N-dimethyl-formamide; for 15h; | To <strong>[101-06-4]N,N-dibenzyl-2-aminoethanol</strong> (80.68 g) were added cyclohexane (500 ml) and DMF (12.9 ml), and thereto was added dropwise thionyl bromide (83.4 g). The mixture was stirred for 15 hours, and to the reaction solution was added an saturated aqueous sodium hydrogen carbonate solution in an ice-bath, and the mixture was extracted with ethyl acetate. The organic layer was washed with water (three times) and saturated aqueous sodium chloride solution, dried over sodium sulfate, filtered and concentrated under reduced pressure to give the title compound (72.1 g). 1H NMR (400 MHz, CDCl3) delta 7.48-7.39 (m, 8H), 7.36-7.33 (m, 2H), 3.74 (s, 4H), 3.43 (m, 2H), 2.97 (m, 2H). |
[2]Tetrahedron Letters,2008,vol. 49,p. 1648 – 1651.
[3]Patent: US2504977,1946, .
[4]Acta Poloniae Pharmaceutica,1955,vol. 12,p. 223,229.
Chem.Abstr.,1956,p. 12042.
[5]Journal of the Chemical Society,1961,p. 1291 – 1297.
[6]Patent: EP2447264,2012,A1 .Location in patent: Page/Page column 178.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 83% | With potassium carbonate; In ethanol; for 16h;Heating / reflux; | Example 1; Dibenzylaminoethanol:; To a 250 mL round bottom flask equipped with stirbar, reflux condensor, nitrogen inlet, and septum was added potassium carbonate (56.5 g, 0.41 mol), ethanol (80 mL), and ethanolamine (9.9 mL, 0.16 mol). The stirred solution was heated to reflux and benzyl chloride (37.9 mL, 0.33 mol) was added via syringe. The resulting solution was stirred for 16 hours at reflux, allowed to cool, then poured into water (200 mL). The solution was extracted with chloroform (3×300 mL). The combined organic layers were dried with magnesium sulfate, filtered, then evaporated to dryness. Recrystallization from hexanes yielded the product as colorless crystals (32.8 g, 83 %). 1H NMR (300 MHz, CDCl3) delta 7.33 (10 H), 3.60 (4 H), 3.58 (2 H), 2.67 (2H). 13C NMR (75 MHz, CDCl3) delta 138.9, 129.2, 128.6, 127.4, 58.7, 58.4, 55.0. |
| 69% | With potassium carbonate; In ethanol; for 36h;Reflux; | Dibenzylamino Ethanol Benzyl chloride (278.5g, 2.2 mol), ethanol amine (60 mL, 1 mol), potassium carbonate (283.1g, 2.05mol) and ethanol (2 L) were mixed together in a 3L 3- neck flask, fitted with an overhead stirrer, a condenser and a glass plug. The apparatus was heated up to reflux for 36 hr, after which the insoluble solid was filtered through a medium frit. The filtrate was recovered and ethanol was removed by rotoary evaporation. The viscous liquid was redissolved in ether, the solid suspension removed by filtration and extracted twice against water. The ether solution was kept and the aqueous layer was extracted twice with dichloromethane (2 x 400 mL). The fraction were recombined, dried over MgSO4, stirred over carbon black for 15 min and filtered through a celite pad. Dichloromethane was removed and the solid was redissolved into a minimal amount of ether (combined volume of 300 mL with the first ether fraction, 300 m
Details |








Reviews
There are no reviews yet.