Cat.NO.:A172077 Purity:98%
Product Details of [ 100858-33-1 ]
| CAS No. : | 100858-33-1 |
| Formula : |
C12H15NO3 |
| M.W : |
221.25
|
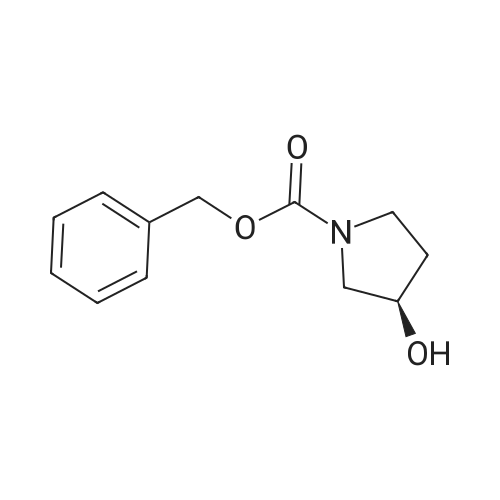
| SMILES Code : | O=C(N1C[C@H](O)CC1)OCC2=CC=CC=C2 |
| MDL No. : | MFCD07368258 |
| InChI Key : | MBLJFGOKYTZKMH-LLVKDONJSA-N |
| Pubchem ID : | 11183628 |
Safety of [ 100858-33-1 ]
| GHS Pictogram: |  |
| Signal Word: | Danger |
| Hazard Statements: | H301-H315-H319-H335 |
| Precautionary Statements: | P261-P301+P310-P305+P351+P338 |
| Class: | 6.1 |
| UN#: | 2811 |
| Packing Group: | Ⅲ |
Computational Chemistry of [ 100858-33-1 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 16 |
| Num. arom. heavy atoms | 6 |
| Fraction Csp3 | 0.42 |
| Num. rotatable bonds | 4 |
| Num. H-bond acceptors | 3.0 |
| Num. H-bond donors | 1.0 |
| Molar Refractivity | 62.98 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
49.77 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
2.35 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
1.13 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
0.86 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
1.14 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
1.13 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
1.32 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-1.94 |
| Solubility | 2.56 mg/ml ; 0.0116 mol/l |
| Class?
Solubility class: Log S scale |
Very soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-1.77 |
| Solubility | 3.77 mg/ml ; 0.017 mol/l |
| Class?
Solubility class: Log S scale |
Very soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-2.14 |
| Solubility | 1.61 mg/ml ; 0.00726 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-6.85 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
0.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
2.63 |
Application In Synthesis of [ 100858-33-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100858-33-1 ]
[ 100858-33-1 ] Synthesis Path-Downstream 1~7
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 89% | Sodium trifluoroacetate (32.6 g) was suspended in toluene (213 g), and DFI (32.6 g) was added dropwise thereto at 0C. The mixture was stirred at 0C for 1 hour, and then R-CHP (44.2 g) was added thereto. The reaction solution was stirred at 0C for 1 hour, and then at 50C for 2 hours. The reaction solution was kept at 15C or lower, a 5 wt% aqueous sodium hydrogen carbonate solution (480 g) was added dropwise to the reaction solution, which was stirred at room temperature for 30 min. Subsequently, the toluene layer was separated. The toluene layer was washed three times with water (100 g), and then concentrated under reduced pressure. To the obtained residue were added toluene (40 g) and hexane (80 g), and the mixture was crystallized at 0C. The obtained crystal was collected by filtration, and dried at 40C for 12 hours under reduced pressure to obtain a target compound. Amount 39.3 g Yield 89% Stereoisomer ratio S-CHP:R-CHP=99.9:0.1 1H-NMR (toluene-d8, 400 MHz) delta 7.23-7.20 (m, 2H), 7.13-6.98 (m, 3H), 5.11-4.99 (m, 2H), 3.93 (bs, 1H), 3.46-3.12 (m, 4H), 1.65-1.45 (m, 1H), 1,45-1.30 (m, 1H) The stereoselectivity in the reaction was determined by using the concentrated residue before crystallization and using the peak area ratio of S-CHP and R-CHP under an HPLC analysis condition 6. S-CHP:R-CHP=99.8:0.2 | |
| 85% | Sodium trifluoroacetate (2.04 g) was suspended in toluene (10 g), and PPDA (3.35 g) was added dropwise thereto at 0C. The mixture was stirred at 0C for 1 hour, and then R-CHP (1.11 g) was added thereto. The reaction solution was stirred at 0C for 1 hour, and then at 50C for 2 hours. To the reaction solution was added toluene (20 g), and the mixture was kept at 15C or lower, and added dropwise to an ice-cooled, 5 wt% aqueous sodium hydrogen carbonate solution (30 g). The reaction solution was stirred at room temperature for 30 min, and then the toluene layer was separated. The toluene layer was washed three times with water (20 g), and then concentrated under reduced pressure to obtain a residue as a pale brown solid containing a target compound. Amount 0.94 g Yield 85% The stereoselectivity in this reaction was determined from the peak area ratio of S-CHP and R-CHP under an HPLC analysis condition-6. Stereoisomer ratio S-CHP:R-CHP=96:4 1H-NMR (toluene-d8, 400 MHz) was consistent with that in Example 29. | |
| 78% | Sodium trifluoroacetate (2.04 g) was suspended in toluene (10 g), and BDDF (2.07 g) was added dropwise thereto at 0C. The mixture was stirred at 0C for 1 hour, and then R-CHP (1.11 g) was added thereto. The reaction solution was stirred at 0C for 1 hour, and then at 50C for 2 hours. To the reaction solution was added toluene (20 g), and the mixture was kept at 15C or lower, and added dropwise to an ice-cooled, 5 wt% aqueous sodium hydrogen carbonate solution (30 g). The reaction solution was stirred at room temperature for 30 min, and then the toluene layer was separated. The toluene layer was washed three times with water (20 g), and then concentrated under reduced pressure to obtain a residue as a pale brown solid containing a target compound. Amount 0.87 g Yield 78% The stereoselectivity in this reaction was determined from the peak area ratio of S-CHP and R-CHP under an HPLC analysis condition-6. Stereoisomer ratio S-CHP:R-CHP=94:6 1H-NMR (toluene-d8, 400 MHz) was consistent with that in Example 29. |
[2]Patent: EP2039680,2009,A1 .Location in patent: Page/Page column 44.
[3]Patent: EP2039680,2009,A1 .Location in patent: Page/Page column 45-46.
[4]Bioorganic and Medicinal Chemistry Letters,2004,vol. 14,p. 1265 – 1268.
[5]Journal of Organic Chemistry,2001,vol. 66,p. 8513 – 8517.
[6]Patent: WO2004/43940,2004,A1 .
[7]Patent: WO2005/108382,2005,A1 .
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With sodium hydroxide; In water; | a) Preparation of benzyl (S)-3-hydroxy-pyrrolidine-1-carboxylate The pH of a solution of 6.18 g of (S)-3-hydroxy-pyrrolidine hydrochloride in 175 ml of water was adjusted to 10 with 10% sodium hydroxide solution and cooled to 0-5 C. 7.1 ml of benzyl chloroformate were added dropwise within 30 minutes under argon, with the pH of the solution being held between 9.5 and 11.5 by the dropwise addition of 10% sodium hydroxide solution. After completion of the addition the suspension was stirred at room temperature for 16 hours. The suspension was extracted with ethyl acetate, the organic phase was washed with water, dried over Na2 SO4, filtered and the filtrate was concentrated. Purification of the crude product over a silica gel column gave 7.33 g of benzyl (R)-3-hydroxy-pyrrolidine-1-carboxylate as a beige liquid. |











Reviews
There are no reviews yet.