Cat.NO.:A128553 Purity:97%
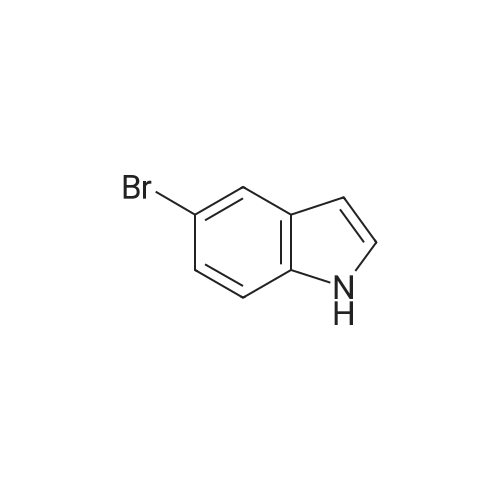
Product Details of 5-Bromoindole
| CAS No. : | 10075-50-0 |
| Formula : |
C8H6BrN |
| M.W : |
196.04
|
| SMILES Code : | C1=CC(=CC2=C1[NH]C=C2)Br |
| MDL No. : | MFCD00005670 |
| InChI Key : | VXWVFZFZYXOBTA-UHFFFAOYSA-N |
| Pubchem ID : | 24905 |
Safety of 5-Bromoindole
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of 5-Bromoindole
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 10075-50-0 ]
- Downstream synthetic route of [ 10075-50-0 ]
[ 10075-50-0 ] Synthesis Path-Downstream 1~28
- 1
 [ 10075-50-0 ]
[ 10075-50-0 ]
 [ 199609-62-6 ]
[ 199609-62-6 ]
- [3-(1H-Indol-5-yl)-benzyl]-carbamic acid tert-butyl ester [ No CAS ]
References: [1]Tetrahedron Letters,1998,vol. 39,p. 4467 – 4470.
References: [1]Bioorganic and Medicinal Chemistry Letters,2007,vol. 17,p. 1793 – 1798.
[2]European Journal of Medicinal Chemistry,2001,vol. 36,p. 545 – 553.
[3]Chemical Biology and Drug Design,2011,vol. 77,p. 182 – 188.
[4]European Journal of Medicinal Chemistry,2015,vol. 99,p. 125 – 137.
[5]Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,p. 3024 – 3028.
[6]ChemMedChem,2016,vol. 11,p. 1446 – 1458.
[7]RSC Advances,2016,vol. 6,p. 30412 – 30424.
[8]European Journal of Medicinal Chemistry,2018,vol. 156,p. 722 – 737.
[9]Organic Letters,2019,vol. 21,p. 7702 – 7707.
[2]European Journal of Medicinal Chemistry,2001,vol. 36,p. 545 – 553.
[3]Chemical Biology and Drug Design,2011,vol. 77,p. 182 – 188.
[4]European Journal of Medicinal Chemistry,2015,vol. 99,p. 125 – 137.
[5]Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,p. 3024 – 3028.
[6]ChemMedChem,2016,vol. 11,p. 1446 – 1458.
[7]RSC Advances,2016,vol. 6,p. 30412 – 30424.
[8]European Journal of Medicinal Chemistry,2018,vol. 156,p. 722 – 737.
[9]Organic Letters,2019,vol. 21,p. 7702 – 7707.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With NaH; phosphoric acid; In tetrahydrofuran; N,N-dimethyl-formamide; | 1-(2-Hydroxyethyl)-5-bromoindole: Under a N2 atmosphere, 2-bromoethanol (17.6 g, 141 mmol) and 2-methoxypropene (10.1 g, 141 mmol) were stirred in 71 mL of THF at 0 C. for 30 minutes The resulting solution was added to a stirring mixture of 5-bromoindole (22.83 g, 116 mmol) and 60% NaH (4.62 g, 193 mmol) in 40 mL of DMF and 60 mL of THF. The solution was stirred at ambient temperature for 4 hours. The reaction mixture was worked up by quenching the excess of NaH with water and removing the aqueous layer. The organic layer was vigorously stirred with 200 mL of 2% aqueous phosphoric acid for 5 hours when the layers were separated. The organic layer was washed with water (2*200 mL) and the solvent removed. The residue was purified by column chromatography (30:70 EtOAc-Hex) to give 17 g of 1-(2-hydroxylethyl)-5-bromoindole. Tris[1-(2-t-Butyldimethylsilyloxyethyl)indol-5-yl]bismuthane: | |
| With NaH; phosphoric acid; In tetrahydrofuran; N,N-dimethyl-formamide; | 1-(2-Hydroxyethyl)-5-bromoindole: Under a N2 atmosphere, 2-bromoethanol (17.6 g, 141 mmol) and 2-methoxypropene (10.1 g, 141 mmol) were stirred in 71 mL of THF at 0 C. for 30 minutes The resulting solution was added to a stirring mixture of 5-bromoindole (22.83 g, 116 mmol) and 60% NaH (4.62 g, 193 mmol) in 40 mL of DMF and 60 mL of THF. The solution was stirred at ambient temperature for 4 hours. The reaction mixture was worked up by quenching the excess of NaH with water and removing the aqueous layer. The organic layer was vigorously stirred with 200 mL of 2% aqueous phosphoric acid for 5 hours when the layers were separated. The organic layer was washed with water (2*200 mL) and the solvent removed. The residue was purified by column chromatography (30:70 EtOAc-Hex) to give 17 g of 1-(2-hydroxylethyl)-5-bromoindole. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With sodium hydroxide; In dimethyl sulfoxide; | STEP 7A 1-(2-Hydroxyethyl)-5-bromoindole A mixture of NaOH (4.4 gm, 0.011 mol) in DMSO (175 ml) was stirred at 100 C. for 5 hours at which time it was cooled to 20 C. To this mixture was added 5-bromoindole (20 gm, 0.102 mol) and the reaction was stirred for 8 hours at room temperature. A solution of ethylene oxide (5.1 gm, 0.125 mol) in DMSO (20 ml) was prepared by bubbling the gas into DMSO. To the bromoindole reaction mixture was slowly added the ethylene oxide solution and stirring was continued for another 2.5 hours. The reaction mixture was then poured into ice water and extracted twice with diethyl ether. The combined ether extracts were concentrated in vacuo whereupon crystallization took place. The crude product was recrystallized from diethyl ether:hexanes (3:2) to afford the title compound (6.25 gm). | |
| With sodium hydroxide; In dimethyl sulfoxide; | STEP 1A 1-(2-Hydroxyethyl)-5-bromoindole A mixture of NaOH (4.4 gm, 0.011 tool) in DMSO (175 ml) was stirred at 100 C. for 5 hours at which time it was cooled to 20 C. To this mixture was added 5-bromoindole (20 gm, 0.102 mol) and the reaction was stirred for 8 hours at room temperature. A solution of ethylene oxide (5.1 gm, 0.125 mol) in DMSO (20 ml) was prepared by bubbling the gas into DMSO. To the bromoindole reaction mixture was slowly added the ethylene oxide solution and stirring was continued for another 2.5 hours. The reaction mixture was then poured into ice water and extracted twice with diethyl ether. The combined ether extracts were concentrated in vacuo whereupon crystallization took place. The crude product was recrystallized from diethyl ether:hexanes (3:2) to afford the title compound (6.25 gm). | |
| With sodium hydroxide; In dimethyl sulfoxide; | STEP 25A 1-(2-Hydroxyethyl)-5-bromoindole A mixture of NaOH (4.4 gm, 0.011 mol) in DMSO (175 ml) was stirred at 100 C. for 5 hours at which time it was cooled to 20 C. To this mixture was added 5-bromoindole (20 gm, 0.102 mol) and the reaction was stirred for 8 hours at room temperature. A solution of ethylene oxide (5.1 gm, 0.125 mol) in DMSO (20 ml) was prepared by bubbling the gas into DMSO. To the bromoindole reaction mixture was slowly added the ethylene oxide solution and stirring was continued for another 2.5 hours. The reaction mixture was then poured into ice water and extracted twice with diethyl ether. The combined ether extracts were concentrated in vacuo whereupon crystallization took place. The crude product was recrystallized from diethyl ether:hexanes (3:2) to afford the title compound (6.25 gm). |
| With sodium hydroxide; In dimethyl sulfoxide; | STEP 25A 1-(2-Hydroxyethyl)-5-bromoindole A mixture of NaOH (4.4 gm, 0.011 mol) in DMSO (175 ml) was stirred at 100 C. for 5 hours at which time it was cooled to 20 C. To this mixture was added 5-bromoindole (20 gm, 0.102 mol) and the reaction was stirred for 8 hours at room temperature. A solution of ethylene oxide (5.1 gm, 0.125 mol) in DMSO (20 ml) was prepared by bubbling the gas into DMSO. To the bromoindole reaction mixture was slowly added the ethylene oxide solution and stirring was continued for another 2.5 hours. The reaction mixture was then poured into ice water and extracted twice with diethyl ether. The combined ether extracts were concentrated in vacuo whereupon crystallization took place. The crude product was recrystallized from diethyl ether:hexanes (3:2) to afford the title compound (6.25 gm). |

| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 66% | With dmap; triethylamine; In 1,2-dichloro-ethane; at 20 – 60℃; for 8h; | General procedure: Triethylamine (3.8 mmol, 1.5 equiv), acetic anhydride (9.6 mmol, 3.8 equiv) and DMAP(0.5 mmol, 0.2 equiv) were added at rt to a solution of 5-bromo-1H-indole (2.5 mmol, 1 equiv) in1,2 dichloroethane (10 mL). The solution was heated to 60 C for 8 h. When the reaction wascomplete [TLC (EtOAc/hexane 2:5)], the mixture was extracted with EtOAc (2 × 20 mL) andwashed with water (2 × 20 mL). The organic layer was separated, dried over anhydrous Na2SO4,filtered and the solvent removed under reduced pressure. The residue was purified by flashcolumn chromatography [silica gel (230-400 mesh; Merck), EtOAc/hexane 2:5]. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 46% | With copper(l) iodide; potassium carbonate; lithium chloride; In N,N-dimethyl-formamide; at 120℃; for 64h;Inert atmosphere; | Description 205-bromo-1-(3-fluoro-pyridin-4-yl)-1H-indole (D20)5-bromo-1H-indole [CA.S. 10075-50-0] (2 g, 10.202 mmol) was dissolved in DMF (16 ml). A nitrogen stream was bubbled through the mixture and then were added 3-Fluoro- 4-iodopyridine [CA.S. 22282-75-3] (2.502 g, 11.222 mmol), lithium chloride (0.432 g, 10.202 mmol), copper(I) iodide (0.0195 g, 0.102 mmol) and K2CO3 (4.23 g, 30.605 mmol). The reaction mixture was heated at 120 0C for 2 days. After cooling to room temperature, the reaction mixture was refilled with lithium chloride (0.100 g) and (copper(I) iodide (0.010) stirred at 1200C for 16 h. After cooling to room temperature, the reaction mixture was washed with NH3 (aqueous sat. solution) and extracted with DCM. The organic layer was separated, washed with water, dried (Na2SO4), and the solvent was evaporated in vacuo. The residue was purified by column chromatography (silica gel; eluent: Heptane/EtOAc up to 15percent as eluent). The desired fractions were collected and the solvent was evaporated in vacuo to yield intermediate compound D20 (1.37 g, 46 percent). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 79% | With tetra-(n-butyl)ammonium iodide; zinc trifluoromethanesulfonate; N-ethyl-N,N-diisopropylamine; In toluene; at 20℃;Inert atmosphere; | General procedure: Procedure A: To a solution of 1H-indole-5-sulfonamide/5-(methylsulfonyl)-1H-indole (2 equiv.), zinc triflate (1.2 equiv.) and tetrabutylammonium iodide (1 equiv.) in dry toluene (ca. 3 mL per mmol of indole) was added N,N-diisopropylethylamine (2.2 equiv.) under argon. The reaction mixture was heated at 50 °C for 30 min, followed by addition of the p-halo-benzyl bromide (1 equiv.). The mixture was stirred overnight at 50 °C under argon. The reaction was quenched with saturated aqueous NH4Cl, diluted with distilled water and extracted with ethyl acetate. The combined organic layers were washed with water and brine, dried over sodium sulfate anhydrous, filtered and concentrated. The samples were further purified with silica flash chromatography then PTLC. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With lithium tert-butoxide; In N,N-dimethyl-formamide; at 100℃; under 760.051 Torr; for 24h; | General procedure: In a dried two-necked test tube was charged with LiOtBu (160 mg, 2.00 mmol) and indole 1a (23.4 mg, 0.4 mmol). The reaction vessel was evacuated under high vacuum and the atmosphere was replace with a balloon of CO2. Then DMF (2 mL) was added and the mixture was stirred for 24 h at 100C. Then the result mixture was cooled and carefully quenched with a solution of HCl (2 N) and extracted with EtOAc (5x). The combined organic layers were washed with water (2x), brine (1x) and dry over MgSO4. The dried organics were concentrated under reduce pressure and the residue was purified by preparative TLC (hexane:acetone = 1:1) to afford the desired product 2a (153.0 mg, 95%) as a white solid. |
References: [1]Patent: US2012/277205,2012,A1 .
[2]Patent: WO2012/146318,2012,A1 .
[3]Patent: EP2548864,2013,A1 .
[4]Patent: WO2013/14102,2013,A1 .
[5]Journal of Medicinal Chemistry,2014,vol. 57,p. 7293 – 7316.
[6]Journal of Medicinal Chemistry,2017,vol. 60,p. 2745 – 2763.
[7]Russian Journal of General Chemistry,2017,vol. 87,p. 3006 – 3016.
[8]Chemical Papers,2018,vol. 72,p. 1369 – 1378.
[9]Bioorganic and Medicinal Chemistry,2019,vol. 27,p. 1043 – 1055.
[10]Journal of Molecular Structure,2020,vol. 1208.
[2]Patent: WO2012/146318,2012,A1 .
[3]Patent: EP2548864,2013,A1 .
[4]Patent: WO2013/14102,2013,A1 .
[5]Journal of Medicinal Chemistry,2014,vol. 57,p. 7293 – 7316.
[6]Journal of Medicinal Chemistry,2017,vol. 60,p. 2745 – 2763.
[7]Russian Journal of General Chemistry,2017,vol. 87,p. 3006 – 3016.
[8]Chemical Papers,2018,vol. 72,p. 1369 – 1378.
[9]Bioorganic and Medicinal Chemistry,2019,vol. 27,p. 1043 – 1055.
[10]Journal of Molecular Structure,2020,vol. 1208.
Be the first to review “5-Bromoindole” 取消回复














Reviews
There are no reviews yet.