Cat.NO.:A166328 Purity:97%
Product Details of [ 1006-64-0 ]
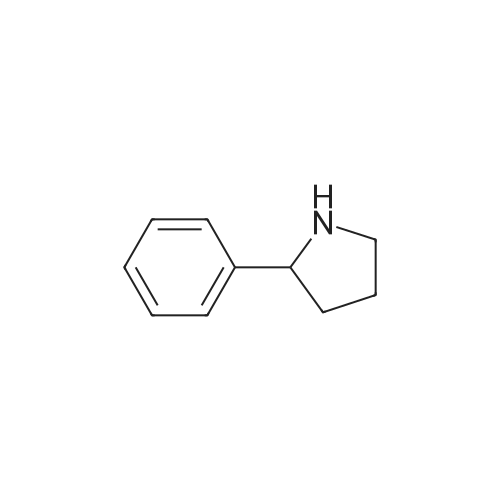
| CAS No. : | 1006-64-0 |
| Formula : |
C10H13N |
| M.W : |
147.22
|
| SMILES Code : | C1CNC(C1)C1=CC=CC=C1 |
| MDL No. : | MFCD01631835 |
| InChI Key : | JUTDHSGANMHVIC-UHFFFAOYSA-N |
| Pubchem ID : | 261892 |
Safety of [ 1006-64-0 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H332-H335 |
| Precautionary Statements: | P261-P280-P305+P351+P338 |
Application In Synthesis of [ 1006-64-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1006-64-0 ]
[ 1006-64-0 ] Synthesis Path-Downstream 1~35
References: [1]Tetrahedron Letters,1994,vol. 35,p. 2775 – 2778.
References: [1]Organic Letters,2005,vol. 7,p. 4329 – 4331.
References: [1]Organic Letters,2007,vol. 9,p. 4123 – 4126.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
- 20
- 4-methyl-benzenesulfinic acid (2-benzenesulfonyl-1-phenyl-but-3-enyl)-amide [ No CAS ]

 [ 1006-64-0 ]
[ 1006-64-0 ]
References: [1]Tetrahedron Letters,2000,vol. 41,p. 8157 – 8162.
References: [1]Tetrahedron Letters,1994,vol. 35,p. 2775 – 2778.
- 28
 [ 796600-15-2 ]
[ 796600-15-2 ]
 [ 1006-64-0 ]
[ 1006-64-0 ]
- 2-chloro-3-methyl-4-(2-phenyl-pyrrolidin-1-yl)-benzonitrile [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 37% | With 4-methyl-morpholine; at 150℃; | Heat <strong>[796600-15-2]2-chloro-4-fluoro-3-methyl-benzonitrile</strong> (144 mg, 0.85 mmol) and 2- . phenyl-pyrrolidine (0.15 g, 1.02 mmol, 1.20 equivalents) in N-methylmorpholine (0.11 ml, 1.02 mmol, 1.20 equivalents) at 150 0C overnight. Allow the reaction mixture to cool to room temperature, dilute with dichloromethane (1 ml), load on silica, and purify by chromatography (12 g silica gel, 0 to 100% ethyl acetate/hexanes over 20 minutes) to obtain 150 mg of an oily residue. Recrystallize from ethyl acetate/hexanes to obtain the title compound (92 mg, 37%). LCMS(APCI+): 297.0 (M+l)+; 1H NMR (400 MHz, CDCl3): delta 7.25 (s, 2H), 7.19 (m, 2H), EPO <DP n=”58″/>6.65 (d, IH), 4.70 (m, IH), 4.06 (m, IH), 3.18 (m, IH), 2.44 (m, IH), 2.43 (s, 3H), 2.12 (m, IH), 1.94 (m, 2H). |
References: [1]Chemical Communications,2010,vol. 46,p. 222 – 224.













Reviews
There are no reviews yet.