Cat.NO.:A194236 Purity:98%
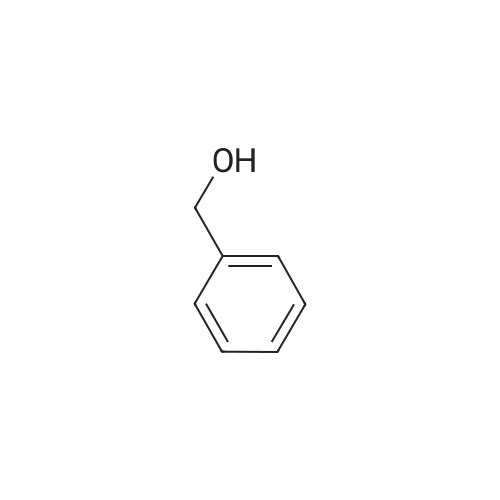
Product Details of Benzyl alcohol
| CAS No. : | 100-51-6 |
| Formula : |
C7H8O |
| M.W : |
108.14
|
| SMILES Code : | OCC1=CC=CC=C1 |
| Synonyms : |
Benzenemethanol
|
| MDL No. : | MFCD00004599 |
| InChI Key : | WVDDGKGOMKODPV-UHFFFAOYSA-N |
| Pubchem ID : | 244 |
Safety of Benzyl alcohol
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of Benzyl alcohol
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 100-51-6 ]
- Downstream synthetic route of [ 100-51-6 ]
[ 100-51-6 ] Synthesis Path-Upstream 1~14
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 89% | Stage #1: With sodium hydride In N,N-dimethyl-formamide; mineral oil at 5℃; for 0.25 h; Stage #2: at 5 – 60℃; |
A flask is charged with NaH (60percent dispersion in mineral oil, 0.72 g, 18.0 mmol) then suspended in DMF (30 mL) and cooled to 5° C. To this mixture is added benzyl alcohol (1.04 mL, 10.0 mmol) drop-wise. The mixture is stirred for 15 minutes then 4-chloropyridine-HCl (1.00 g, 6.67 mmol) is added in three portions over 5 min. The resulting mixture is stirred at 5° C. for 10 min then warmed to 60° C. and stirred for 1.5 h. The mixture is then cooled to 23° C., treated with water, and extracted with EtOAc. The combined organics are dried with MgSO4, filtered, and concentrated in vacuo. Purification of the crude by flash chromatography (SiO2, 5percent EtOAc in hexanes to 50percent EtOAc in hexanes) gives the title intermediate (1.10 g, 89percent). |
| 89% | Stage #1: With sodium hydride In N,N-dimethyl-formamide; mineral oil at 5℃; for 0.25 h; Stage #2: at 5 – 60℃; for 1.75 h; |
Example 3 Synthesis of 4-(benzyloxy)pyridine. A flask is charged with NaH (60percent dispersion in mineral oil, 0.72 g, 18.0 mmol) then suspended in DMF (30 mL) and cooled to 5 °C. To this mixture is added benzyl alcohol (1.04 mL, 10.0 mmol) drop-wise. The mixture is stirred for 15 minutes then 4-chloropyridine-HCl (1.00 g, 6.67 mmol) is added in three portions over 5 min. The resulting mixture is stirred at 5 °C for 10 min then warmed to 60 °C and stirred for 1.5 h. The mixture is then cooled to 23 °C, treated with water, and extracted with EtOAc. The combined organics are dried with MgSO4, filtered, and concentrated in vacuo. Purification of the crude by flash chromatography (SiO2, 5percent EtOAc in hexanes to 50percent EtOAc in hexanes) gives the title intermediate (1.10 g, 89percent). |
[1] Journal of Organic Chemistry, 2017, vol. 82, # 24, p. 13756 – 13767.
[2] Patent: US2011/275627, 2011, A1, . Location in patent: Page/Page column 60.
[3] Patent: EP2331541, 2011, A1, . Location in patent: Paragraph 0070; 0071.
[1] Tetrahedron, 1987, vol. 43, # 11, p. 2557 – 2564.
[2] Patent: WO2010/90716, 2010, A1, . Location in patent: Page/Page column 301.
[1] Tetrahedron, 2002, vol. 58, # 24, p. 4931 – 4935.
[1] Tetrahedron Letters, 1998, vol. 39, # 32, p. 5685 – 5688.
[1] Heterocycles, 1996, vol. 42, # 1, p. 265 – 272.
[1] Journal of the Chemical Society, Chemical Communications, 1994, # 19, p. 2301 – 2302.
[1] Synthetic Communications, 2004, vol. 34, # 1, p. 33 – 39.
[1] Journal of Organic Chemistry, 1992, vol. 57, # 15, p. 4243 – 4249.
[2] Patent: WO2004/31156, 2004, A1, . Location in patent: Page 30.
[3] Patent: US2005/54707, 2005, A1, . Location in patent: Page/Page column 12.
[1] Organometallics, 2014, vol. 33, # 16, p. 4269 – 4278.
[1] Bioorganic and Medicinal Chemistry, 2010, vol. 18, # 17, p. 6220 – 6229.
[1] Organic Letters, 2012, vol. 14, # 5, p. 1206 – 1209.
[1] Organic and Biomolecular Chemistry, 2018, vol. 16, # 44, p. 8537 – 8545.







































Reviews
There are no reviews yet.