Cat.NO.:A288778 Purity:99%
Product Details of [ 100-27-6 ]
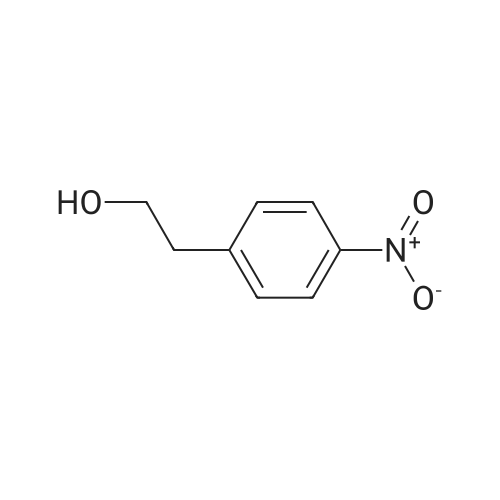
| CAS No. : | 100-27-6 |
| Formula : |
C8H9NO3 |
| M.W : |
167.16
|
| SMILES Code : | O=[N+](C1=CC=C(C=C1)CCO)[O-] |
| MDL No. : | MFCD00010202 |
| InChI Key : | IKMXRUOZUUKSON-UHFFFAOYSA-N |
| Pubchem ID : | 7494 |
Safety of [ 100-27-6 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H319 |
| Precautionary Statements: | P264-P270-P280-P301+P312+P330-P305+P351+P338-P337+P313-P501 |
Computational Chemistry of [ 100-27-6 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 12 |
| Num. arom. heavy atoms | 6 |
| Fraction Csp3 | 0.25 |
| Num. rotatable bonds | 3 |
| Num. H-bond acceptors | 3.0 |
| Num. H-bond donors | 1.0 |
| Molar Refractivity | 46.2 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
66.05 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
1.43 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
1.12 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
1.13 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
1.49 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
-0.16 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
1.0 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-1.75 |
| Solubility | 2.95 mg/ml ; 0.0176 mol/l |
| Class?
Solubility class: Log S scale |
Very soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-2.1 |
| Solubility | 1.33 mg/ml ; 0.00793 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-2.0 |
| Solubility | 1.65 mg/ml ; 0.00989 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-6.52 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
1.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
2.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.5 |
Application In Synthesis of [ 100-27-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 100-27-6 ]
[ 100-27-6 ] Synthesis Path-Downstream 1~7
[2]Bulletin de la Societe Chimique de France,1933,vol. <4> 53,p. 330,332.
[3]Helvetica Chimica Acta,1981,vol. 64,p. 1688 – 1703.
[4]Bulletin de la Societe Chimique de France,1931,vol. <4> 49,p. 3,5.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| In dichloromethane; carbon tetrabromide; | EXAMPLE 4 Preparation of 2,2 di (4-nitrophenyl) ethyl bromide To a stirred solution of 2,2 di (4-nitrophenyl) ethanol (0.155 g, 0.54 mmoles) produced by the process of Example 2 in carbon tetrabromide (1.08 moles, 0.362 g) was added triphenyl phosphine (21.08 mmoles, 0.289 g). The originally clear liquid turned yellow immediately, whilst thin layer chromatography indicated that no starting material was present. The solvent was removed under vacuum to give, after flash chromatography (eluent, 1:4 ethyl acetate: petrol ether), the bromide in crystalline form (yield 75%); recrystallisation was from methylene chloride. |














Reviews
There are no reviews yet.