Cat.NO.:A143500 Purity:98%
Product Details of p-Anisic acid
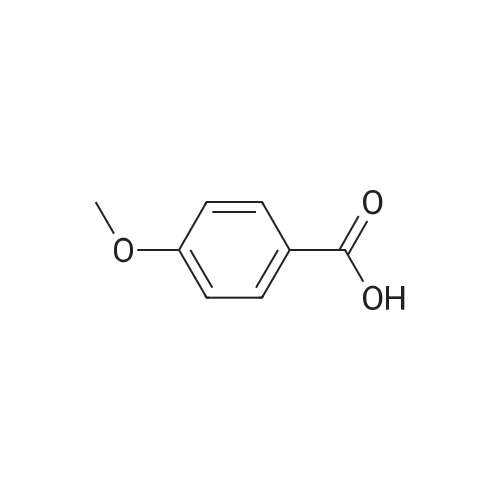
| CAS No. : | 100-09-4 |
| Formula : |
C8H8O3 |
| M.W : |
152.15
|
| SMILES Code : | O=C(O)C1=CC=C(OC)C=C1 |
| Synonyms : |
4-Methoxybenzoic acid; Draconic acid; NSC 32742
|
| MDL No. : | MFCD00002542 |
| InChI Key : | ZEYHEAKUIGZSGI-UHFFFAOYSA-N |
| Pubchem ID : | 7478 |
Safety of p-Anisic acid
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Application In Synthesis of p-Anisic acid
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 100-09-4 ]
- Downstream synthetic route of [ 100-09-4 ]
[ 100-09-4 ] Synthesis Path-Downstream 1~28
References: [1]Tetrahedron Letters,1991,vol. 32,p. 3187 – 3190.
References: [1]Tetrahedron Letters,1981,vol. 22,p. 2785 – 2788.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 73% | With poly phosphoric acid; at 80℃; for 1.5h; | General procedure: In order to prepare PPA (polyphosphoric acid), a mixture of H3PO4 (6.4 g) and P2O5 (11.5 g) in 150 mL beaker was stirred at 80 C for 15 min. 1-Bromo-3-methoxybenzene (9) (3.08 g,16.47 mmol) and 3,4-dimethoxybenzoic acid (7) (2 g, 10.98 mmol) were added to prepared PPA solution and the mixture was stirred with a glass stick at 80 C for 1.5 h. After the completion of the reaction, cold water was added to the reaction mixture and then the organic layer was extracted with EtOAc (3 150 mL). The combined organic phases were dried over Na2SO4 and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography with silica gel (30 g) by eluting with hexane:ethyl acetate (9:1). Recrystallization of the solid with hexane-ethyl acetate gave 2-bromo-4-methoxyphenyl)(3,4-dimethoxyphenyl)methanone (10) (2.32 g) as a white solid with 60% yield. |
References: [1]Archiv der Pharmazie,1906,vol. 244,p. 436.
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 91% | With dimethyl amine; In ethanol; isopropyl alcohol; | a) 200 kg of 2-methoxyethyl acetoacetate and 185.1 kg of 3-nitrobenzaldehyde are suspended in 800 l of isopropanol. Then 5.65 kg of p-anisic acid and 5.05 kg of 33percent dimethylamine in ethanol are added, heating for about 30′ at about 35° C. to obtain a solution. The reaction mixture is left to cool at 20/25° C. and then it is cooled for about 12 hours with running water and for a further 24 hours at about 0° C. with brine, then is centrifuged, washing with isopropanol. After drying, 327 kg of 2-methoxyethyl 2-(3-nitrobenzylidene) acetoacetate are obtained, in an about 91percent yield. |
References: [1]Patent: US6015906,2000,A .
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With 1,1′-carbonyldiimidazole; In propan-1-ol; water; | EXAMPLE 20 The procedure is similar to that described in Example 1, but starting with 4-methoxybenzoic acid (4.55 g), N,N’-carbonyldiimidazole (6.55 g) and <strong>[15992-83-3]2-amino-1,8-naphthyridine</strong> (5.2 g). The product produced by precipitation in water (8.35 g; m.p. 85 C., viscous) is filtered and then dissolved in boiling 1-propanol (50 cc). After 1 hour’s cooling at 4 C., the crystallized solid is separated by filtration, washed with 1-propanol (2*5 cc) and dried at 35 C. under reduced pressure (0.067 kPa). N-(1,8-naphthyridin-2-yl)-4-methoxybenzamide (6.9 g) is produced, m.p. 150 C. 2-Amino-1,8-naphthyridine can be prepared according to U.S. Pat. No. 3,948,917. |
References: [1]Patent: US4642308,1987,A .
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With PPA; at 180℃; for 2h; | Example 143 Amethyl 2-(4-methoxyphenylbenzo[d]oxazole-7-carboxylate; 4-Methoxybenzoic acid (152 mg, 1.0 mmol) and <strong>[17672-21-8]methyl 2-amino-3-hydroxybenzoate</strong> (167 mg, 1.0 mmol) were added to PPA (1 g), the mixture was stirred at 180 C. for 2 hr. After cooled to room temperature, water (50 mL) was added and filtered and the cake was washed with water and dried in vacuum to give methyl 2-(4-methoxyphenylbenzo[d]oxazole-7-carboxylate. LC-MS (ESI) m/z 240 [M+1]+. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 71.8% | To a 100 mL flask was added (4-methoxybenzoic acid)(976 mg, 6.41 mmol) and acetonitrile (11 mL) were added and dissolved,85% Phosphoric Acid (0.125 mL, 6.41 mmol) and trifluoroacetic anhydride (3 mL, 21.39 mmol) were added and stirred for 10 minutes.Then, <strong>[348-37-8]ethyl-6-fluoro-1H-indole-2-carboxylate</strong> (1.1 g, 4.28 mmol) synthesized in the above Step 2 was added,Stir for 10 hours.After completion of the reaction, the organic layer was separated using ethyl acetate and water, and the organic layer was further treated with saturated sodium hydrogencarbonate and sodium chloride, and water was removed with magnesium sulfate.After filtration, the mixture was purified by column chromatography (EA / n-Hex = 1: 4) to give ethyl 6-fluoro-3- (4- methoxybenzoyl) -1H-indole- Rate(1.2 g, 3.06 mmol, 71.8%). |
- 25
 [ 100-09-4 ]
[ 100-09-4 ]
Details
Be the first to review “p-Anisic acid” 取消回复

























Reviews
There are no reviews yet.