Cat.NO.:A417797 Purity:97%
Product Details of [ 1000342-95-9 ]
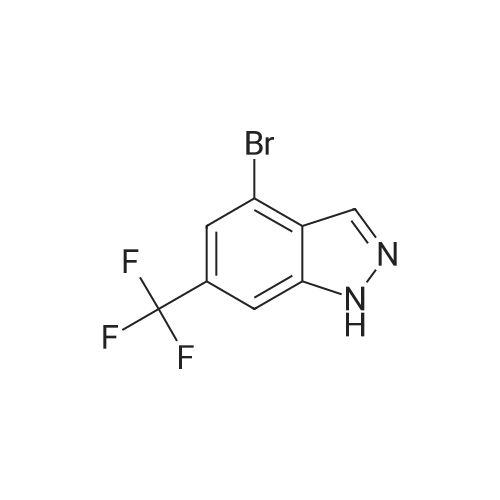
| CAS No. : | 1000342-95-9 |
| Formula : |
C8H4BrF3N2 |
| M.W : |
265.03
|
| SMILES Code : | FC(F)(F)C1=CC(Br)=C2C=NNC2=C1 |
| MDL No. : | MFCD09026956 |
| InChI Key : | JRUCLQINUHVRJW-UHFFFAOYSA-N |
| Pubchem ID : | 24729232 |
Safety of [ 1000342-95-9 ]
| GHS Pictogram: |  |
| Signal Word: | Warning |
| Hazard Statements: | H302-H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
Computational Chemistry of [ 1000342-95-9 ] Show Less
Physicochemical Properties
| Num. heavy atoms | 14 |
| Num. arom. heavy atoms | 9 |
| Fraction Csp3 | 0.12 |
| Num. rotatable bonds | 1 |
| Num. H-bond acceptors | 4.0 |
| Num. H-bond donors | 1.0 |
| Molar Refractivity | 48.8 |
| TPSA ?
Topological Polar Surface Area: Calculated from |
28.68 Ų |
Lipophilicity
| Log Po/w (iLOGP)?
iLOGP: in-house physics-based method implemented from |
1.47 |
| Log Po/w (XLOGP3)?
XLOGP3: Atomistic and knowledge-based method calculated by |
3.13 |
| Log Po/w (WLOGP)?
WLOGP: Atomistic method implemented from |
4.5 |
| Log Po/w (MLOGP)?
MLOGP: Topological method implemented from |
2.9 |
| Log Po/w (SILICOS-IT)?
SILICOS-IT: Hybrid fragmental/topological method calculated by |
3.65 |
| Consensus Log Po/w?
Consensus Log Po/w: Average of all five predictions |
3.13 |
Water Solubility
| Log S (ESOL):?
ESOL: Topological method implemented from |
-3.86 |
| Solubility | 0.0362 mg/ml ; 0.000137 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (Ali)?
Ali: Topological method implemented from |
-3.4 |
| Solubility | 0.105 mg/ml ; 0.000397 mol/l |
| Class?
Solubility class: Log S scale |
Soluble |
| Log S (SILICOS-IT)?
SILICOS-IT: Fragmental method calculated by |
-4.65 |
| Solubility | 0.00592 mg/ml ; 0.0000223 mol/l |
| Class?
Solubility class: Log S scale |
Moderately soluble |
Pharmacokinetics
| GI absorption?
Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant?
BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate?
P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor?
Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
Yes |
| CYP2C19 inhibitor?
Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor?
Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor?
Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor?
Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)?
Skin permeation: QSPR model implemented from |
-5.69 cm/s |
Druglikeness
| Lipinski?
Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose?
Ghose filter: implemented from |
None |
| Veber?
Veber (GSK) filter: implemented from |
0.0 |
| Egan?
Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge?
Muegge (Bayer) filter: implemented from |
0.0 |
| Bioavailability Score?
Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
Medicinal Chemistry
| PAINS?
Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk?
Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness?
Leadlikeness: implemented from |
No; 1 violation:MW<0.0 |
| Synthetic accessibility?
Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.76 |
Application In Synthesis of [ 1000342-95-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1000342-95-9 ]
[ 1000342-95-9 ] Synthesis Path-Downstream 1~35
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 78% | With toluene-4-sulfonic acid; In tetrahydrofuran; for 18h;Inert atmosphere; Reflux; | Referential example 25 (l-(Tetrahydro-2H-pyran-2-yl)-6-(trifluoromethyl)-lH-indazol-4-yl)methanamine (127) step 1 : A round-bottom flask was charged with 4-bromo-6-(trifluoromethyl)-lH-indazole (1.00 g, 3.8 mmol), TsOH monohydrate (27 mg, 0.15 mmol), 3,4-dihydro-2H-pyran (1.59 g, 18.9 mmol), and THF (25 mL). The reaction mixture was degassed with nitrogen and heated under reflux for 18 h, and then the solvent was removed in vacuo. The residue was purified by S1O2 chromatography to afford 4-bromo-l – (tetrahydro-2H-pyran-2-yl)-6-(trifluoromethyl)-lH-indazole (1.03 g, 78percent) as a yellow oil. MS (ESI) m/z: 265.2 [(M – THP group)+l]+. |
- 4
- (4-((2-hydroxyethyl)carbamoyl)phenyl)boronic acid [ No CAS ]

 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
 [ 1454293-91-4 ]
[ 1454293-91-4 ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 30% | EXAMPLE 130: N-(2-hydroxyethyl)-4-(6-(trifiuoromethyl)-lH-indazol-4- yl)benzamide [0550] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.300 g, 1.132 mmol), (4-((2-hydroxyethyl)carbamoyl)phenyl)boronic acid (0.237 g, 1.132 mmol) and PdCl2(dppf) (0.041 g, 0.057 mmol) in dioxane (10 mL) and aqueous saturated NaHC03 (3 mL). The resulting yellow suspension was heated at 140°C for 60 minutes in a microwave reactor. The reaction mixture was subsequently concentrated and the crude residue was purified by preparative HPLC, eluting with 40percent> ACN (containing 0.035percent) TFA) in H20 (containing 0.05percent TFA) over a period of 4.5 minutes. The product-containing fractions were combined and volatiles removed in vacuo to give a TFA salt of the title compound as white solid (0.12 g, 30percent). 1H NMR (400 MHz, CD3OD) delta ppm 3.56 (t, J=5.81 Hz, 2 H), 3.75 (t, J=5.81 Hz, 2 H), 7.49 (d, J=1.01 Hz, 1 H), 7.78-7.89 (m, 2 H), 7.93 (s, 1 H), 8.00-8.10 (m, 2 H), 8.21-8.32 (m, 1 H); ESI-MS m/z [M+H]+ calc’d for Ci7Hi4F3N302, 350.1; found 350.1. |
- 5
 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
 [ 628692-15-9 ]
[ 628692-15-9 ]
- 4-(2-methoxypyrimidin-5-yl)-6-(trifluoromethyl)-1H-indazole trifluoroacetate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 39% | EXAMPLE 246: 4 2-methoxypyrimidin-5-yl)-6-(trifluoromethyl)-lH-indazole [0784] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.1 g, 0.377 mmol), (2-methoxypyrimidin-5-yl)boronic acid (0.076 g, 0.491 mmol) and PdCl2(dppf) (0.014 g, 0.019 mmol) in dioxane (8 mL) and aqueous saturated NaHC03 (2 mL). The resulting light brown suspension was heated at 140°C for 45 minutes in a microwave reactor. The reaction mixture was subsequently concentrated and the crude residue was purified by preparative HPLC, eluting with a gradient of 35-40percent ACN (containing 0.035percent TFA) in H20 (containing 0.05percent TFA) over a period of 8 minutes. The volatiles were removed in vacuo to give a TFA salt of the title compound as white solid (0.043 g, 39percent). 1H NMR (400 MHz, DMSO-<) delta ppm 4.03 (s, 3 H), 7.60 (d, J=1.01 Hz, 1 H), 8.00 (s, 1 H), 8.34-8.52 (m, 1 H), 9.06 (s, 2 H); ESI-MS m/z [M+H]+ calc’d for Ci3H9F3N40, 295.1; found 295.15. |
- 6
 [ 1015480-87-1 ]
[ 1015480-87-1 ]
 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
- 4-(3,6-dimethoxypyridazin-4-yl)-6-(trifluoromethyl)-1H-indazole trifluoroacetate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 4% | EXAMPLE 248 : 4-(3 ,6-dimethoxypyridazin-4-yl)-6-(trifluoromethyl)- lH-indazole [0788] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.15 g, 0.566 mmol),(3,6-dimethoxypyridazin-4-yl)boronic acid (0.135 g, 0.736 mmol) and PdCl2(dppf) (0.021 g, 0.028 mmol) in dioxane (10 mL) and aqueous saturated NaHC03 (3 mL). The resulting light brown suspension was heated at 140°C for 45 minutes in a microwave reactor. The reaction mixture was subsequently concentrated and the crude residue was purified by preparative HPLC, eluting with a gradient of 40-45percent ACN (containing 0.035percent TFA) in H20 (containing 0.05percent TFA) over a period of 6.5 minutes. The volatiles were removed in vacuo to give a TFA salt of the title compound as a light brown solid (8 mg, 4percent). 1H NMR (400 MHz, DMSO-d6) delta ppm 3.97 (s, 3 H), 4.03 (s, 3 H), 7.42 (s, 1 H), 7.55 (d, J=1.01 Hz, 1 H), 8.05 (s, 1 H), 8.14 (s, 1 H), 13.74 (s, 1 H); ESI-MS m/z [M+H]+ calc’d for Ci4HiiF3N402, 325.1; found 325.16. |
- 7
 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
 [ 459856-12-3 ]
[ 459856-12-3 ]
- 4-(6-methoxy-2-methylpyridin-3-yl)-6-(trifluoromethyl)-1H-indazole trifluoroacetate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 47% | EXAMPLE 249: 4-(6-methoxy-2-methylpyridin-3-yl)-6-(trifluoromethyl)-lH- indazole [0790] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.15 g, 0.566 mmol), (6-methoxy-2-methylpyridin-3-yl)boronic acid (0.123 g, 0.736 mmol) and PdCl2(dppf) (0.021 g, 0.028 mmol) in dioxane (10 mL) and aqueous saturated NaHC03 (3 mL). The resulting light brown suspension was heated at 140°C for 45 minutes in microwave reactor. The reaction mixture was subsequently concentrated and the crude residue was purified by preparative HPLC, eluting with 45percent ACN (containing 0.035percent) TFA) in H20 (containing 0.05percent TFA) over a period of 6.5 minutes. The volatiles were removed in vacuo to give a TFA salt of the title compound as a light brown oil (82 mg, 47percent). 1H NMR (400 MHz, OMSO-de) delta ppm 2.30 (s, 3 H), 3.93 (s, 3 H), 6.75-6.86 (m, 1 H), 7.29 (d, J=1.26 Hz, 1 H), 7.72 (d, J=8.34 Hz, 1 H), 7.90-8.03 (m, 2 H); ESI-MS m/z [M+H]+ calc’d for Ci5Hi2F3N30, 308.1; found 308.15. |
- 8
 [ 864754-16-5 ]
[ 864754-16-5 ]
 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
- ethyl2-(4-(6-(trifluoromethyl)-1H-indazol-4-yl)-1H-pyrazol-1-yl)acetate trifluoroacetate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 37% | EXAMPLE 260: ethyl 2-(4-(6-(trifluoromethyl)-lH-indazol-4-yl)-lH-pyrazol-l- yl)acetate [0812] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.3 g, 1.132 mmol), ethyl 2-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazol-l- yl)acetate (0.349 g, 1.245 mmol) and PdCl2(dppf) (0.041 g, 0.057 mmol) in dioxane (10 mL) and aqueous saturated NaHC03 (3 mL). The resulting light brown suspension was heated at 140°C for 45 minutes in a microwave reactor. The reaction mixture was subsequently concentrated and the crude residue was purified by preparative HPLC, eluting with a gradient of 40-45percent ACN (containing 0.035percent TFA) in H20 (containing 0.05percent TFA) over a period of 6.5 minutes. The product-containing fractions were combined and the volatiles were removed via rotary evaporation to give a TFA salt of the title compound as a light brown solid (0.14 g, 37percent). 1H NMR (400 MHz, OMSO-d6) delta ppm 1.24 (t, J=7.07 Hz, 3 H), 4.19 (q, J=7.16 Hz, 2 H), 5.15 (s, 2 H), 7.60 (d, J=1.01 Hz, 1 H), 7.77 (s, 1 H), 8.27 (d, J=0.76 Hz, 1 H), 8.53 (s, 1 H), 8.63 (s, 1 H), 13.64 (s, 1 H); ESI-MS m/z [M+H]+ calc’d for Ci5Hi3F3N402, 339.1; found 339. |
- 9
 [ 1072945-86-8 ]
[ 1072945-86-8 ]
 [ 1000342-95-9 ]
[ 1000342-95-9 ]
 [ 76-05-1 ]
[ 76-05-1 ]
- methyl 5-(6-(trifluoromethyl)-1H-indazol-4-yl)picolinatetrifluoroacetate [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
EXAMPLE 269: methyl 5-(6-(trifluoromethyl)-lH-indazol-4-yl)picolinate [0830] A vial was charged with a mixture of 4-bromo-6-(trifluoromethyl)-lH-indazole (0.732 g, 2.76 mmol), (6-(methoxycarbonyl)pyridin-3-yl)boronic acid (0.5 g, 2.76 mmol) and PdCl2(dppf) (0.101 g, 0.138 mmol) in dioxane (10 mL) and aqueous saturated NaHC03 (3 mL). The resulting light brown suspension was heated at 140°C for 45 minutes in a microwave reactor. The reaction mixture was subsequently concentrated. The residue was diluted with DCM, washed with water, and the volatiles removed via rotary evaporation. The crude product was purified by CombiFlash® chromatography (0-30percent MeOH in DCM over 180 minutes). The product-containing fractions were combined and concentrated by rotary evaporation to give product with some impurities (0.28 g). A portion of the product (20 mg) was re-purified by preparative HPLC, eluting with 40percent ACN (containing 0.035percent TFA) i
Details |



Reviews
There are no reviews yet.